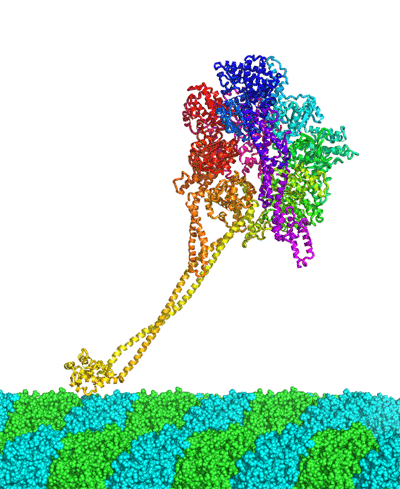
Elucidation of the atomic structure of the motor protein Dynein (Press Release)
- Release Date
- 08 Mar, 2012
- BL44XU (Macromolecular Assemblies)
Osaka University
|
Summary of presentation Structural elucidation of the protein molecule was carried out utilizing the data collected through the use of the beam line BL44XU installed by Osaka University at the SPring-8. The results updated the record of the longest polypeptide chains that have been structurally determined up to now. The study results were published on Advanced Online Publication of the U.K. science journal Nature on the 8th of March (JST), ahead of the normal printed publication. Publication: |
<<Figures>>
|
For more information‚ please contact: |
- Previous Article
- Preparation of Rock-Salt-Type Crystal of Li+-Encapsulated Fullerene C60 and Clarification of Its Structure (Press Release)
- Current article
- Elucidation of the atomic structure of the motor protein Dynein (Press Release)