Elucidation of previously unknown offense-defense mechanism in intestinal tract: pathogenic microbe (Shigella) vs. host’s innate immunity (Press Release)
- Release Date
- 12 Mar, 2012
- BL44XU (Macromolecular Assemblies)
Tokyo University
|
Every year, approximately 15 million people lose their lives due to infectious diseases, and around 2 million out of them are caused by enteral infections. Although the intestinal tract is constantly exposed to numerous microbes, it has layers of protective barriers centered on innate immunity*5 to guard the living system from microbial invasion. On the other hand, the enteropathogenic species Shigella and the allied pathogenic E-coli (typically 157) possess a highly sophisticated mechanism to worm their way into the living body, tactically eluding the protective systems. However, the details of these mechanisms-the innate immunity mechanism blocking pathogenic microbial invasion into intestinal mucosa, and the strategy employed by pathogenic microbes to circumvent them-have been a complete mystery. In more specific terms, the results are summarized as below. Publication: |
<<Figures>>
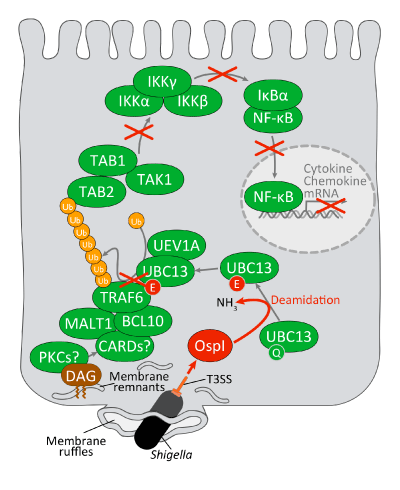
The invasion of Shigella*1 into epithelial cells triggers the formation of a ruffle membrane (leaf-like protrusion; macropinocytosis) in the vicinity of the microbe. The bacteria break the ruffle membrane and finally reach the cellular cytoplasm. As the ruffle membrane formation proceeds in the basolateral surface of epithelial cells, diacylglycerol (DAG) tends to localize in the membrane. Diacylglycerol acts as a second messenger for the signal created by protein kinase C (PKC) and thus has the effect of activating the CMB (CARD-MALT1-BCL10) complex -TRAF6- NF-kB pathway, resulting in induced production of antibacterial peptides, inflammatory cytokine or chemokine.*6 To circumvent this for continued survival and proliferation within the cytoplasm, Shigella secretes an OspI effector (a pathogenic agent) through its type 3 secretion machine*3 when it invades the cell. The OspI makes a specific bonding to UBC13 and converts its 100-th glutamine into a glutamic acid through deamidation, leading to deactivation of the UBC13. As the UBC13 serves as an activating factor of TRAF6, its deactivation hinders the activation of TRAF6, thus disabling the NF-kB signal pathways that lie downstream. Using this scheme, Shigella inhibits the host's inflammation and innate immune reaction immediately after the onset of its infection, resulting in the accelerated proliferation of the pathogenic microbe within the mucosal epithelia.
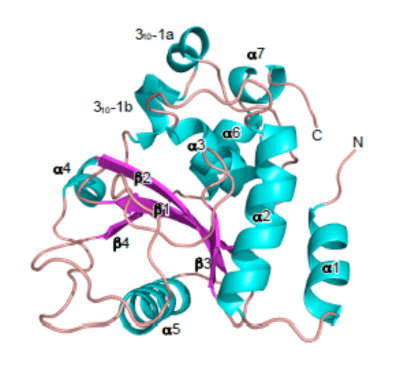
A crystalline sample of OspI protein-secreted through the type 3 secretion machine of Shigella-was successfully prepared and used for crystal structure analysis. The analysis was conducted using SPring-8 (BL44XU), which successfully clarified the steric configuration of OspI in 2.0Å resolution (registered to Protein Data Bank, ID number: 3B21). The analysis proved that OspI had a structure consisting of four β-strands (red), seven α-helixes (blue), and one 310 helix (blue). A comparison of OspI's steric structure with those of other reported proteins in terms of their primary sequences indicated that no known protein had a primary amino acid sequence similar to OspI. However, it revealed that the steric structure of OspI is similar to that of AvrPphB, which is a known etiological agent of Pseudomonas-Syringae (a phytopathogenic fungus with a type 3 secretion machine similar to that of Shigella). The AvrPphB protein is a protease enzyme that has the same type of active center as that of OspI, i.e. a center consisting of cysteine (Csy), histidine (His), and asparagine acid (Asp). By taking advantage of these clues, substitution variants were prepared for each of the amino acids, which then clearly indicated the importance of the three amino-acid residues (i.e. Cys-His-Asp) with respect to Ospl's activity of deamidating UBC13.
<<Glossary>>
*1 Shigella
Shigella (Bacillus dysenteriae) belongs to the same taxonomic class as Escherichia coli, and is the causative microorganism of shigellosis. Last August, a shigella outbreak was triggered in a restaurant chain by an infection of this microorganism. Shigella initiates infection by invading the absorptive epithelial cells for proliferation in the lower intestine (large intestine and rectum), and spreads into the neighboring epidermal cells. The infection is detected by innate immunity, causing inflammation inside the intestinal tract.
*2 Mucosal epithelia
Monolayer epithelial cells on the surface of luminal organs (e.g. intestinal tract) play an important role in the defense mechanism (a barrier against microbial invasions), as well as in the absorption of nourishment from intraluminal surfaces.
*3 Type 3 secretion machine
The pathogenic agent (effector) secretion complex innately present in many causative bacteria.
*4 Diacylglycerol
A molecule consisting of two fatty acid molecules covalently bonded to a glycerol molecule through ester linkage. It is localized in a host cell membrane and acts as a second messenger.
*5 innate immunity
The innate immunity system that naturally exists in our body. It constitutes a defense system peculiar to a living body that recognizes and precludes the invasion of disease-causing agents such as microorganisms.
*6 Inflammatory cytokine, chemokine
Any of the low molecular weight proteins involved in detecting the invasion of disease causing agents (or an incidence of heterogenized body of its own) and sending signals to surrounding cells and tissues, thus triggering an activation of the (inflammatory) immune system. These proteins have the effect of gathering neutrophils and hematopietic cells to the inflammation site.
|
For more information‚ please contact: |
- Current article
- Elucidation of previously unknown offense-defense mechanism in intestinal tract: pathogenic microbe (Shigella) vs. host’s innate immunity (Press Release)