Platinum in Fuel Cells -World’s first successful real-time analysis of the reaction mechanism of cobalt alloy catalyst- (Press Release)
- Release Date
- 30 May, 2012
- BL01B1 (XAFS)
- BL40XU (High Flux)
Institute for Molecular Science (National Institutes of Natural Sciences)
Japan Synchrotron Radiation Research Institute (JASRI)
|
The research group led by Associate Professor Mizuki Tada (Institute for Molecular Science) and Dr. Tomoya Uruga (associate chief scientist, JASRI) succeeded for the first time in the world in capturing, in real time, a platinum-cobalt alloy catalyst actually functioning as a cathode (positive electrode) catalyst of a fuel cell system at SPring-8.*1 The fuel cell is now gaining wider use as a next generation energy source-for example as the “Ene-farm” (fuel cell for household use)-as well as being actively promoted toward commercialization in industrial uses, typically for use in vehicles. However, the fuel cell still faces huge challenges: the upgrade of power generation performance, measures against the degradation of costly platinum catalyst, and reduction of the amount of platinum use. Actual fuel systems involve a large amount of water and fuel gases that hinder direct, real-time observation of the electricity generation, making it difficult to understand the structure and reaction mechanism of platinum-based catalyst. The research group focused attention on platinum-cobalt alloy catalyst, which is a fuel cell cathode catalyst known to be superior to platinum catalyst in terms of electricity generation performance and anti-degradation properties, but the origin of these are not yet known. The research group made the first attempt to capture the dynamic process of catalyst reactions and structural changes. The fast time-resolved XAFS*2 method developed at SPring-8, one of the best performing methods in the world, was used to capture the process at 500ms resolution while the fuel cell is actually in operation. The research results shed light on the reaction mechanism taking place on cathode surfaces and identified one of the factors controlling elution-degradation of platinum-cobalt alloy catalyst. The research was conducted as part of a NEDO project (Demonstrative Research on Solid Oxide Fuel Cells / Fundamental Technologies Development / Analysis of Structure, Reactions, and Material Transfer of MEA Materials), and the results were published in the online version of “ACS Catalysis” (a publication by the American Chemical Society) on May 30 (EST). Publication: |
<<Figures>>
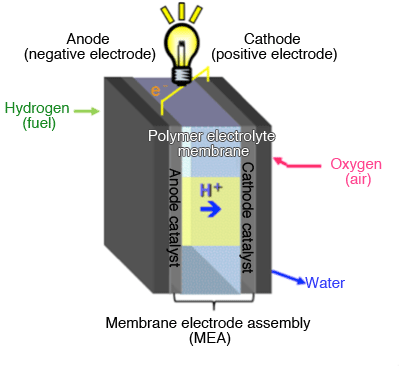
Catalyst is used in both of the electrodes (anode and cathode).
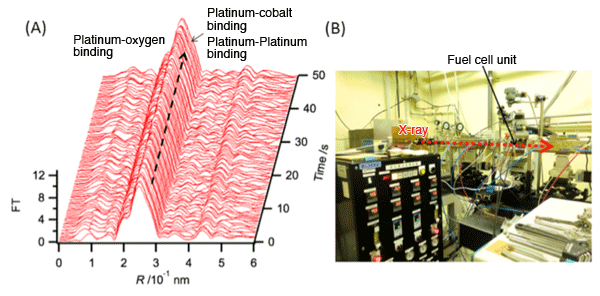
(A) High speed time-resolved XAFS spectra of fuel cell electrode (platinum-cobalt alloy catalyst). They represent a successful direct capturing of structural changes occurring in platinum-cobalt catalyst at 500ms intervals. (B) BL01B1 and BL40XU beams at SPring-8 were used in this research. The photograph shows the high speed time-resolved XAFS system (BL40XU beam) for measuring the fuel cell.
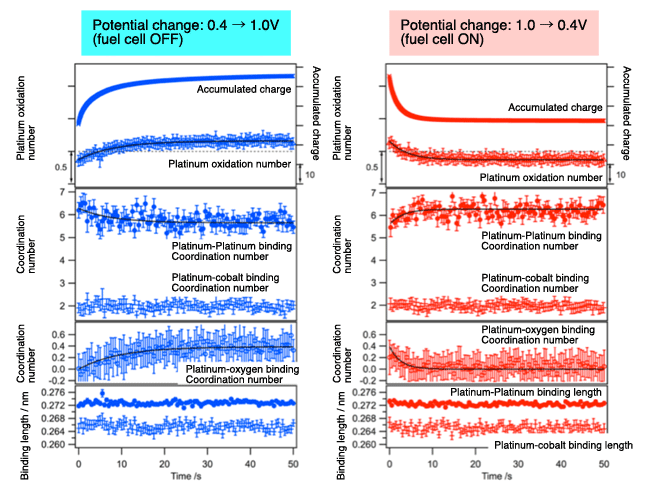
Time changes in catalyst structural parameters (platinum-cobalt alloy catalyst) as acquired through analysis of time-resolved XAFS spectra. Changes of the following parameters with time: quantity of electricity stored in the fuel cell, state of platinum oxidization, coordination number and bonding length of platinum-platinum, platinum-cobalt, and platinum-oxygen bindings.
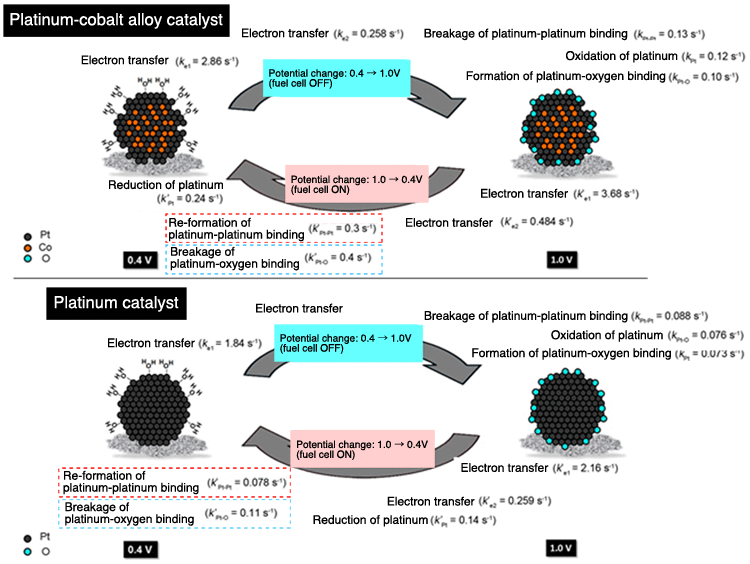
Aspects of reactions on cathode surfaces (platinum-cobalt alloy and platinum catalyst) as revealed by time-resolved XAFS analysis. In comparison to the cases with platinum catalysts, the series of cathode reactions taking place on platinum-cobalt catalysts tend to be quicker: in particular, the binding realignments that occur when the applied potential is returned to 0.4V are observed to be significantly quicker, i.e. the breaking of platinum-oxygen bindings and regeneration of platinum-platinum bindings. As the degradation of the platinum catalyst that accompanies metallic elution can be suppressed by promoting these binding realignments-breakage of platinum-oxygen binding and regeneration of platinum-platinum binding-these realignment processes are highly likely to play a significant part in inhibiting elution degradation of platinum catalyst.
<<Glossary>>
*1 SPring-8
A RIKEN facility located in Harima Science Garden City (Hyogo prefecture) is capable of producing the world's highest intensity synchronous radiation. The management and promotion of utilization of this facility are undertaken by JASRI. The name “SPring-8” comes from “Super Photon ring-8GeV.” An electron flying at nearly the speed of light, if deflected from its original trajectory through the effect exerted by a magnet, emits an electromagnetic wave in a direction tangential to its trajectory, which is called radiation light (or synchrotron radiation). At present, there are three “3rd Generation” large scale synchronous radiation facilities in the world: SPring-8 (Japan), APS (USA) and ESRF (France). The acceleration energy available at SPring-8 (8 billion electron volts) enables the provision of an extremely wide spectrum of radiation light: from far infrared to visible, vacuum ultraviolet, and soft X-ray up to hard X-ray. SPring-8 provides a theater for collaborative works involving researchers inside and outside Japan, and the research conducted at this facility cover such diverse areas as material science, geoscience, life science, environmental science, and various applications in industrial sectors.
*2 XAFS
An acronym for X-ray Absorption Fine Structure. Irradiation of a specific energy region of synchrotron X-ray radiation on a substance provides an X-ray absorption spectrum, a detailed analysis of which, in the neighborhood of the absorption edge (XANES: X-ray Absorption Near-Edge Structure), provides useful information on the symmetry and valency state of the target element. In addition, examination of a wider range of spectrum (EXAFS: eXtended X-ray Absorption Fine Structure) provides clues for determining local coordination structure, i.e. how many and what types of atoms are coordinated at what geometrical distances around the target element. XAFS represents an almost unique technique to probe and determine the substances that lack long-range order, typically catalysts, and if used with hard X-ray emission, it enables in-situ structural analysis under actual catalytic reaction conditions.
*3 Local coordination structure
Atomic configuration at very close distances from the target element. EXAFS analysis can elucidate such information as the number and types of atoms coordinated to the target element, as well as their mutual distances (bonding length).
|
For more information, please contact: |
- Previous Article
- How a Fungi-derived Antifreeze Protein Works: Elucidation of Molecular Structure and Antifreeze Mechanism (Press Release)
- Current article
- Platinum in Fuel Cells -World’s first successful real-time analysis of the reaction mechanism of cobalt alloy catalyst- (Press Release)