Freely Rotating Water Molecules Trapped inside Carbon Cages (Press Release)
- Release Date
- 10 Dec, 2013
- BL02B1 (Single Crystal Structure Analysis)
Nagoya City University
|
Shinobu Aoyagi (associate professor) of the Graduate School of Natural Sciences, Nagoya City University, clarified that water molecules trapped inside spherical carbon fullerenes*2 rotate almost freely at low temperatures of below -250 °C without freezing. This was achieved in collaboration with scientists of Kyoto University, Tohoku University, Nagoya University, and Japan Synchrotron Radiation Research Institute (JASRI). Water normally freezes at 0 °C. The direction of the water molecules in ice is limited owing to the presence of hydrogen bonds*1 and the molecules cannot rotate freely. In contrast, water molecules in fullerenes were found to rotate almost freely even at low temperatures owing to the absence of hydrogen bonds. The research group also found that the direction of water molecules in fullerenes changes in accordance with the direction of an electric field externally applied to these molecules. It is known that the direction of water running from a faucet is bent in response to static electricity. Similarly, the direction of water molecules in fullerenes was found to change in response to static electricity. Fullerenes that trap water molecules are promising as a clean new material for electronic devices with low environmental impact, because they consist of only water and carbon and do not require rare-earth elements, in addition to the above-mentioned electrical properties. The results were published in Chemical Communications, a journal published by the Royal Society of Chemistry, on 18 January 2014. Prior to this, they were published online on 10 December 2013. Publication: |
<<Figures>>
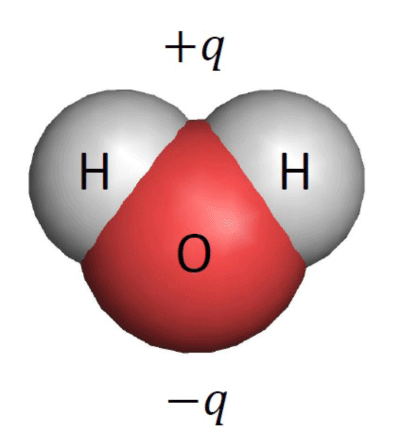
Electrons (-q) are distributed in the oxygen atom (red) more than in the hydrogen atoms (white). Because of this electrical polarity, water molecules respond to static electricity.
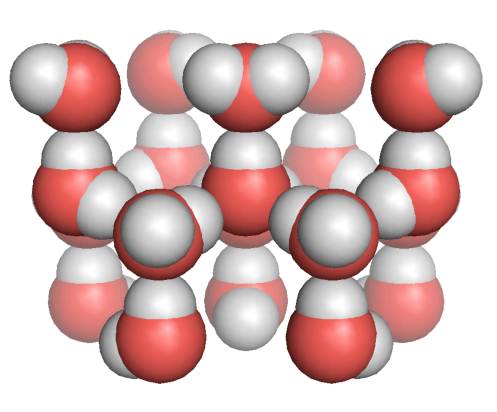
Hydrogen atoms face oxygen atoms in the neighboring water molecules owing to hydrogen bonding. The direction of the water molecules is strongly restricted by hydrogen bonding.
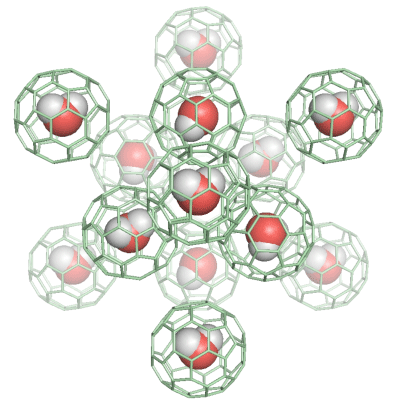
The cages drawn in green are fullerene molecules. The water molecules in fullerenes do not form hydrogen bonding and rotate rapidly even at low temperatures.
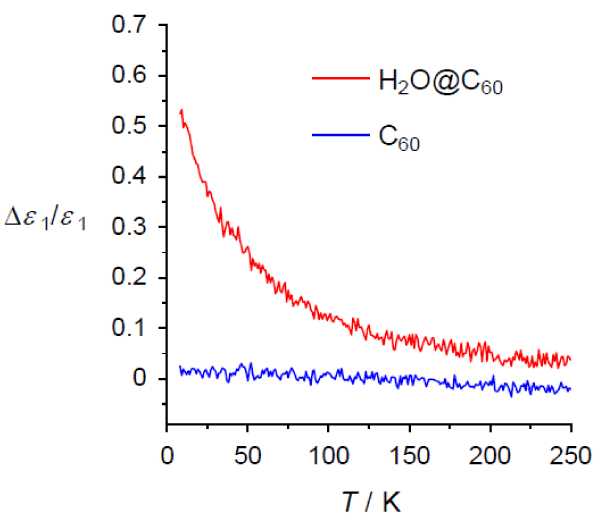
The changes in the permittivity (Δε1) of H2O@C60 and C60 relative to each permittivity at room temperature are plotted against absolute temperature T (0 K = -273 °C and 273 K = 0 °C). The permittivity of H2O@C60 increases with decreasing temperature.
<<Glossary>>
*1 Hydrogen bond
Hydrogen bonding is a phenomenon in which atoms that are covalently bonded with hydrogen atoms are attracted to other atoms via hydrogen atoms. Hydrogen atoms that are covalently bonded with highly electronegative atoms, such as nitrogen and oxygen atoms, are positively charged and form hydrogen bonds through electrostatic attraction to surrounding atoms. Hydrogen bonds are much weaker than covalent and ionic bonds but play an important role in the formation of proteins and deoxyribonucleic acids (DNAs) in vivo.
*2 Fullerene, C60
A fullerene is a hollow spherical soccer-ball-shaped molecule consisting of 60 carbon (C) atoms. Fullerenes were discovered by Harold Kroto and his colleagues, who received the Nobel Prize in Chemistry for their discovery. The mass production of C60 fullerenes has become possible; they are used as a material in sporting equipment, cosmetics, and solar cells. Fullerenes can trap metal atoms, gas molecules, or water molecules inside their hollow spheres.
*3 Permittivity
Permittivity is a constant representing the magnitude of the response of a material to an electric field. When an electric field is applied to a material, a biased distribution of electric charges is induced in the material to reduce the electric field. Permittivity indicates the degree of bias in the distribution of electric charges and varies among different materials. The higher the permittivity, the easier a biased distribution is induced and the more charges are stored in the material.
|
For more information, please contact: |
- Previous Article
- Intense two-color double X-ray laser pulses: a powerful tool to study ultrafast processes (Press Release)
- Current article
- Freely Rotating Water Molecules Trapped inside Carbon Cages (Press Release)