Clarification of Mechanism Underlying Activation of Bacterial Flagellar Motor by Conformational Change of Stator -PomBc “stands” to work and “crouches” to rest- (Press Release)
- Release Date
- 02 Sep, 2014
- BL32XU (RIKEN Targeted Proteins)
- BL41XU (Structural Biology I)
Nagoya University
Osaka University
Key points
• The crystal structure of the C-terminal fragment of PomB (PomBc), the region responsible for anchoring the stator unit of the flagellar motor of Vibrio to the cell wall, was solved using a Spring-8 beamlines BL32XU and BL41XU, and its compact conformation was revealed.
• The compact structure of PomBc indicates thate a part of PomBc changes its conformation upon forming the functional stator.
• Motility of Vibrio cells was impaired when the conformational change of PomBc was inhibited. A part of PomBc is likely to extend when the stator is incorporated into the motor, whereas it shrinks when the stator is dissociated from the motor. This conformational change is considered to be coupled with the activation and deactivation of the motor.
|
A joint research group clarified the mechanism underlying the activation of the bacterial flagellar motor by examining its crystal structure and functions. When the stator responsible for energy conversion is incorporated into the motor, part of the folded stator extends to bind to the cell wall, activating the motor. The research group was led by Shiwei Zhu (research scientist), Seiji Kojima (associate professor), and Michio Homma (professor) of the Graduate School of Science, Nagoya University, and Katsumi Imada (professor) of the Graduate School of Science, Osaka University. Publication: |
《参考図》
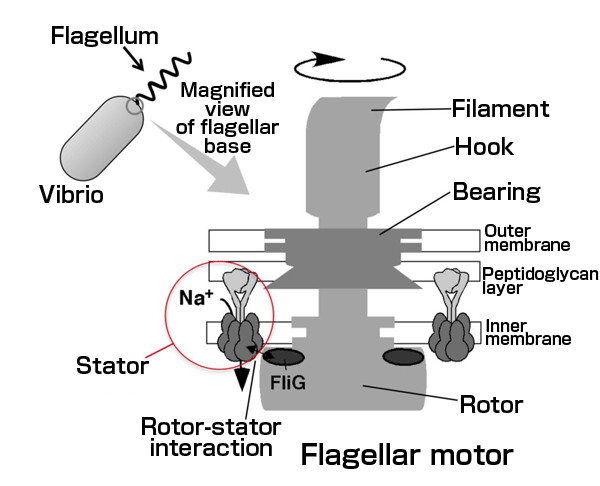
Vibrio has a flagellum at the end of the cell body and swims by rotating the flagellum to thrust a cell like a screw. The engine that generates a torque (flagellar motor, shown in the right-hand magnified view) is located at the base of the flagellum and comprises the stator units and a rotor, which are the main components of the rotary motor, as well as a bearing that supports the rotation of the rotor, a hook acting as a free joint, and a filament that functions as a screw. Multiple stators are located around the rotor. When sodium ions pass through the stators, they interact with the rotor to generate torque.
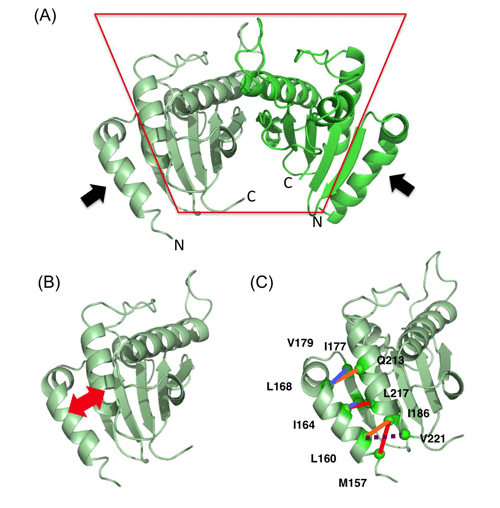
(A) Analyzed structure of PomBc. Two PomBc molecules form a dimer. The region enclosed by the red line represents the region bound to the cell wall, and the black arrows represent the regions expected to change their conformation. N and C indicate the N (amino)- and C (carboxy)-terminals, respectively.
(B) The region indicated by the red arrows were cross-linked to inhibit the conformational change in the experiment.
(C)Pairs of cross-linked amino acids. The motor works even when the residues indicated by the blue lines are cross-linked; however, the motor does not work when the residues indicated by the red lines are cross-linked. The residues indicated by the orange lines are partly cross-linked and lose the motor function. The residues indicated by the dotted line are not cross-linked and maintain the motor function. Therefore, the motor function is lost when N-terminal two-thirds of this helix, as indicated by the black arrows in (A), is fixed by cross-linking.
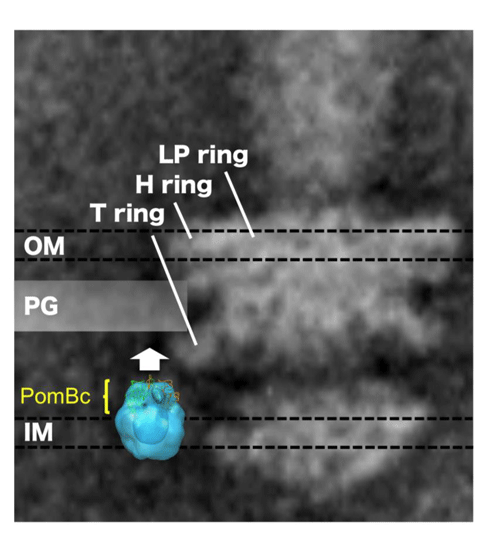
The PomBc structure is superposed onto an electronic microscopic image of the stator and shown with an electron microscopic image of the flagellar base. OM, PG, and IM represent the outer membrane, peptidoglycan layer (cell wall), and inner membrane (cell membrane), respectively. PomBc with this structure does not reach the peptidoglycan layer (cell wall). To reach the layer, PomBc must extend in the direction indicated by the white arrow.
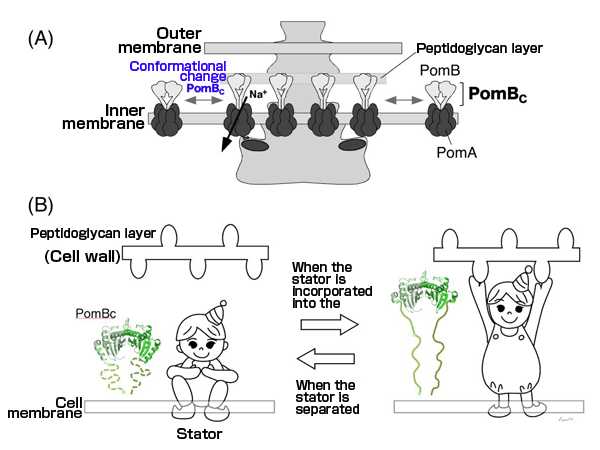
(A) Schematic diagram of the conformational change.
(B) Illustration to explain the conformational change. When the stator is incorporated into the motor, PomBc changes its conformation, as if it has “stood up”, and binds to the cell wall. When the stator is separated from the motor, PomBc does not reach the cell wall, as if is “crouching”; hence, PomBc does not bind to the cell wall and freely moves on the cell membrane.
《Glossary》
Vibrio
A type of bacterium. The marine Vibrio used in this study inhabits the sea and is nontoxic. Pathogenic Vibrio cholerae and Vibrio parahaemolyticus are related species of marine Vibrio.
Flagellum
A bacterial motility organ that works as a screw. A flagellum is extended from the cell body. The number of flagella depends on the type of bacterium.
Stator
A complex responsible for the flow of ions to convert energy in the flagellar motor. The stator unit is anchored to the cell wall (the peptidoglycan layer) and does not rotate. Approximately ten stators are located around the rotor. When ions pass through the stator, it interacts with the rotor to generate torque.
Rotor
The rotational region of the flagellar motor. The rotor is embedded in the cell envelope and comprises multiple rings and a rod that penetrates the rings. The rod is connected to the flagellar filament via a hook that acts as a universal joint. In the rotor, rotation of the rod is supported by the ring structure, thereby they function as a bearing.
Disulfide cross-linking
A biochemical technique for the intramolecular or intermolecular cross-linking of proteins via covalent bonds. An amino acid called cysteine, which is a constituent of proteins, has a highly reactive sulfhydryl group. In a cell, molecules are cross-linked via disulfide bonds formed between two sulfhydryl groups.
For more information, please contact: Professor Katsumi Imada (Press) |
- Previous Article
- Clarification of Mechanism Underlying Destruction of Red Blood Cells by Pathogenic Bacteria (Press Release)
- Current article
- First Observation of Electronic Structure in Ag-Rh Alloy Nanoparticles Having Hydrogen Absorbing/Storage Property –Attempting to solve the mystery of why Ag-Rh alloy nanoparticles have a similar property to Pd– (Press Release)