Topic 25: Researching Battery Materials
Developing Solar Batteries and Fuel Cells at the Nanolevel
Fossil fuels, including oil, are limited resources, and a lifestyle that fully depends on them cannot last forever. Moreover, there are concerns that the increases in emissions of air pollutants and carbon dioxide could worsen environmental problems. Thus, solar light, wind power, and tidal energy are gaining attention as alternative energy resources, and consequently, the developments of fuel cells have been energetically pursued around the world. The high analyzing power of SPring-8 has consistently produced numerous achievements in fundamental studies to develop various alternative energy resources. In this topic, research achievements realized at SPring-8 on the nanolevel toward new materials for solar batteries as well as elucidating the mechanisms of fuel cells at atomic and electronic levels are reported.
Designing Organic Semiconductors Exhibiting Liquid Crystal Properties
Although a solar battery is an ideal power generating system, its drawbacks include high manufacturing cost and low energy conversion efficiency. Organic semiconductors have been anticipated to overcome these difficulties. Unlike silicon semiconductors, which require expensive and complicated manufacturing processes, organic semiconductors are light and easily manufactured. Thus, there is hope the organic semiconductors will be more affordable. However, obtaining materials with a prominent charge transportation property is difficult.
Dr. Takuzo Aida (Professor, The University of Tokyo, Japan) and colleagues have focused their attention on a fused porphyrin copper complex as a promising candidate as an organic semiconductor. This organic molecule has properties similar to chlorophyll, i.e., it can generate electricity by absorbing a large quantity of light. Many of conductive polymers, which are candidates for organic semiconductors, have a structure where double and single bonds are alternatively arranged. This arrangement is called π-conjugation. Through this structure, the connection between electrons becomes smooth, producing conductivity. A large portion of a fused porphyrin copper complex is occupied by this π-conjugated structure, which is related to its prominent electron transport capability.
However, the hardness of organic semiconductor materials composed of solid crystals makes it difficult to manufacture large-area thin films. On the other hand, soft materials such as liquid crystals have superior manufacturability, but inferior electron transport capabilities. Thus, the development of organic semiconductors that combine high manufacturability and superior electron transport capabilities is demanded. “We were inspired by soap. Soap forms a highly ordered structure from spontaneously hydrophilic and hydrophobic portions,” explains Dr. Aida. Dr. Aida and colleagues hypothesized that a fused porphyrin copper complex could maintain a liquid crystalline state though it is organic matter by implementing both hydrophilic and hydrophobic properties so that the complex is simultaneously water-soluble and water-insoluble.
In April 2006, their research group designed a new molecule with an amphipathic property by introducing hydrophilic side chains on one side of a fused porphyrin copper complex molecule and hydrophobic side chains on the other. This complex was initially heated to 120 °C and then cooled to room temperature. As a consequence, this new molecule spontaneously aggregates to form a column shape (cylindrical shape), and exhibits a liquid crystalline state. Compared to other liquid crystal materials, the electron transport speed of this molecule at room temperature is ten times faster.
How does this molecule combine a prominent electron transport capability and liquid crystallinity? Dr. Aida speculated that the array structure of this molecule is critical. After he consulted with Dr. Masaki Takata (Chief Scientist, the RIKEN SPring-8 Center, Japan) and Dr. Sono Sasaki (Senior Scientist, the Japan Synchrotron Radiation Research Institute), his research team conducted an experiment using a 2D small angle scattering method at SPring-8. The 2D small angle scattering method irradiates X-rays onto a sample to visualize the nanolevel structures. Their experiment revealed that the hydrophilic and hydrophobic phases are separated by a 3-4 nm gap (nm = 10-9 m) (Fig. 1). Thus, integrating a fused porphyrin copper complex into a column shape through the amphipathic property can gain prominent electron transport capability.
This research result is the world's first remarkable accomplishment in this field, and was published in the Journal of the American Chemical Society (October 2008), which is the highest-ranked journal in chemistry.
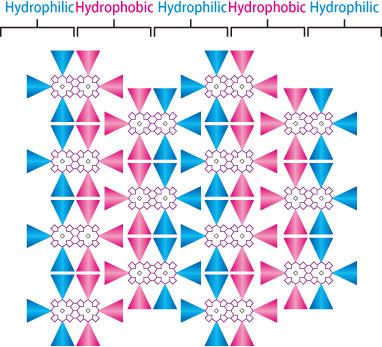
Cross-view of columns (cylindrical structures) formed perpendicular to the plane. Adding hydrophilic side chains (blue triangles) and hydrophobic side chains (red triangles) implements an amphipathic property in fused porphyrins (purple), and yields a structure that exhibits a high electron transportation capability.
Elucidating Mechanisms of Fuel Cells at the Atomic Level
Commercialization of vehicles powered by hydrogen fuel cells, which produce electricity from hydrogen and emit only water, has been eagerly anticipated. Although many issues must be resolved, including enhancing the cathode electromotive force and preventing catalyst degradation due to elution of platinum (Pt), the most serious hurdle to commercialization is the high cost due to the extensive use of Pt. (Pt is one of the most expensive elements naturally found on Earth.) To overcome this serious issue, Dr. Yasuhiro Iwasawa1) (Professor, The University of Tokyo, Japan), Dr. Mizuki Tada2) (Assistant Professor, ditto), and researchers from Toyota Motor Corporation, Toyota Central R&D Labs., High Energy Accelerator Research Organization, and Tottori University formed a research team in April 2005.
“A catalyst superior to platinum is currently unavailable for fuel cells. Even platinum, which is expensive and very durable, degrades with use. Thus, the catalyst itself needs to be further developed. Hence, we aimed to elucidate the electrochemical reactions on the surface of the catalyst at the atomic level during fuel cell operations,” explains Dr. Tada.
The X-ray absorption fine structure (XAFS) technique analyzes the structures of this size of sample. Upon exposure to X-rays, atoms absorb X-rays and emit electrons (photoelectrons). The wave of the photoelectron emitted from an atom is scattered by neighboring atoms and the waves interfere, causing a specific oscillatory structure, called extended XAFS (EXAFS) oscillation, in the X-ray absorption spectrum. Analyses of such oscillations can provide information about the distance and the number of Pt-Pt and Pt-O bonds. However, each sample takes about ten minutes to measure because each energy point in the XAFS technique is individually measured, and these measurements should be repeated at various energies. Thus, real-time changes in the catalytic reactions could not be followed.
To overcome this difficulty, energy dispersive XAFS (DXAFS) and quick XAFS (QXAFS) techniques have been developed by several research groups. In the former, synchrotron radiation containing X-rays with various wavelengths is focused to irradiate onto a sample and spectrum data are captured in one shot. The latter is a technique that allows real-time measurements of a sample by quickly moving a spectroscope, which disperses X-rays of various wavelengths. Dr. Tada's research group developed a new measurement technique based on QXAFS.
Through their technique and full utilization of highly brilliant X-rays to detect small dynamic movements, they successfully observed the chronological changes in fuel cell catalysts with a 1-sec time resolution. They are the first to observe dynamic oxidation-reduction reactions of cathode platinum nanocatalysts under the actual working conditions of a fuel cell, revealing the reaction mechanisms induced by voltage changes during battery operations occur on the surfaces of cathode catalysts (Fig. 2). A particularly important finding is that when the battery cell voltage exceeds the open-circuit voltage, oxygen atoms begin to enter the platinum catalysts, causing catalyst dissolution (Fig. 3). This incursion of oxygen atoms is thought to be the major cause of deterioration of platinum cathode catalysts.
“By actually observing catalyst reactions using synchrotron radiation, the reaction mechanisms of fuel cells have been elucidated for the first time. This experiment has yielded clues to resolve the deterioration issue of cathode catalysts for the commercialization of fuel cells,” explains Dr. Tada. Their research results were published in Angewandte Chemie (May 2007), and researchers around the globe are building upon their results to reduce platinum usage in fuel cells and to develop alternative materials.
1) Currently Professor at the University of Electro-Communications, Japan.
2) Currently Associate Professor at Institute for Molecular Science, National Institutes of Natural Sciences, Japan.
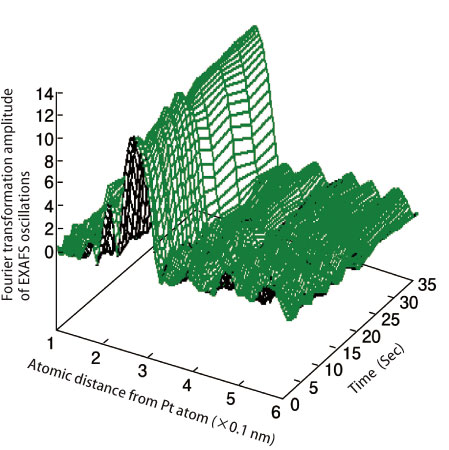
Structural changes of platinum catalysts per second at one of the absorption edges. This data revealed the number of oxygen bonding to platinum increases with time. Measurements were performed using a time-gating quick X-ray absorption fine structure technique, which was developed in collaboration with SPring-8.
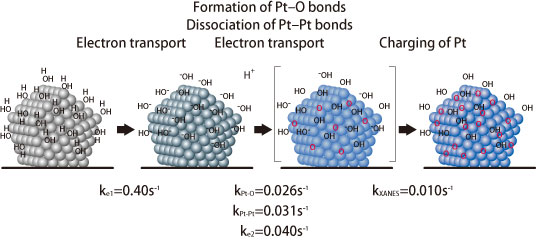
When the battery cell voltage exceeds the open-circuit voltage, oxygen atoms begin to enter the platinum catalysts, triggering platinum dissolution, which leads to catalyst dissolution.
References
1. T. Sakurai, K. Shi, H. Sato, K. Tashiro, A. Osuka, A. Saeki, S. Seki, S. Tagawa, S. Sasaki, H. Masunaga, K. Osaka, M. Takata and T. Aida; J. Am. Chem. Soc., 130, 13812 (2008)
2. M. Tada, S. Murata , T. Asakoka , K. Hiroshima , K. Okumura, H. Tanida, T. Uruga, H. Nakanishi, S. Matsumoto, Y. Inada, M. Nomura and Y. Iwasawa; Angewandte Chemie, 119, 4388 (2007)