Topic 8: Structural Analyses of Supermolecular Assemblies using Synchrotron Radiation Small-Angle X-ray Scattering (SAXS)
Revealing the Properties of Nanoparticles to Realize Drug Delivery Systems
The technology to precisely administer the desired quantity of a pharmaceutical, to the desired part of a living body at the desired time is called a drug delivery system (DDS). DDSs can increase the efficacy of pharmaceuticals, while reducing their side effects. Hence, DDSs are innovative in establishing safe drug therapy systems. Nanotechnology plays a central role in DDSs because nanoparticles as ultrafine capsules that can pass through small holes in blood capillaries and cell membranes need to be fully controlled. To this end, SPring-8's capability has been utilized to reveal the structures of nanoparticles.
Precisely Analyzing the Behaviors of Nanoparticles
To realize highly functional DDSs, nanoscale capsules on the order to 10-100 nm (1 nm = 10-9 m) need to be able to encapsulate and deliver drugs to desired sites through small holes in blood capillaries and cell membranes. The most promising candidates for next generation DDSs are polymeric micelles and micelles composed of lipids.
What are micelles? For example, surfactants such as soap have a configuration similar to marking pins used in sewing where the needle tip corresponds to a lipophilic or hydrophobic group that becomes attached to oil, and the needle head corresponds to a hydrophilic group that becomes attached to water. Materials with such a configuration, when dissolved in water and the concentration exceeds some specific value, will associate with each other with the hydrophilic groups facing outward and the hydrophobic groups facing inward. Association is a phenomenon where two or more identical types of molecules bond with each other and behave as if they are a single molecule. A micellar structure is a structure where several of such molecules containing hydrophobic and hydrophilic groups gather to form a specific structure with the hydrophilic groups facing outward and the hydrophobic groups facing inward. A micellar structure, which is composed of polymers containing numerous hydrophobic and hydrophilic groups, forms a double-structure where the shell is comprised of hydrophilic chains and the core contains hydrophobic chains.
Micelles autonomously construct a particle when the concentration and other conditions are appropriately controlled, enabling drugs to be encapsulated in the core. The radii of the constructed particles range between 10-100 nm and can pass through the blood capillary walls, which is a required feature of DDSs. However, to realize DDSs using nanoparticles such as polymeric micelles, various technological hurdles must be overcome. In particular, it is extremely difficult to have the target deliver drugs to only desired sites and to precisely control the release of drugs at the desired site. A complete understanding of the structures of nanoparticles in solution as well as the correlation of nanoparticles and pharmacological effects are necessary to resolve these issues.
Dr. Kazuo Sakurai (Professor, The University of Kitakyushu, Japan) and colleagues, who strive to establish molecular design techniques, have focused on nanointerfaces. “The structures and functions of nanoparticles such as polymeric micelles are controlled by the interactions between the shell and core on nanointerfaces. The development of DDS particles requires the internal structures of particles, the behavior and formation of drugs on hydrophilic and hydrophobic interfaces in DDS particles, and the fusion of biomembranes and DDS particles to be thoroughly examined,” explains Dr. Sakurai. Thus, investigating the interface between the shell and core is essential to their research.
Investigating the Behavior of Drugs Encapsulated in Polymeric Micelles
Dr. Sakurai's research group began experiments to reveal the structures of micelles by examining nanointerfaces at the Structural Biology Beamline II (BL40B2) at SPring-8. In October 2008, this project was approved as part of the Core Research for Evolutional Science and Technology (CREST) program by the Japan Science and Technology Agency (JST).
Small-angle X-ray scattering (SAXS) is the only technique to examine nanosized biomolecules and their aggregates. X-rays with small scattering angles can reveal the structures of samples at the 1-nm to 1-μm level. Although SAXS allows the configuration of these particles to be visualized on such small scales, the sample concentration of these particles needs to be more than 100 times higher than that in the body because the data signals are very small. Hence, the observed structures would substantially differ from those in the living body condition.
The principal reason why such a high concentration is required is parasitic scattering, which is the optical noise produced from the air around a sample upon irradiation of X-rays. “To conduct experiments using ultra-diluted solutions with the lowest possible particle concentration, which is close to the living body condition as possible, parasitic scattering had to be eliminated,” mentions Dr. Sakurai when explaining the progress of his experiments. To this end, his group developed an innovative device to measure sealed cell encapsulating sample solutions in a vacuum chamber. This system drastically improved valid data (signal: S) to noise such as the parasitic scattering (noise: N) ratio (S/N ratio) (Fig. 1). Regardless of the experimental difficulties, the highly brilliant X-rays at BL40B2 and the RIKEN Structural Biology Beamline I (BL45XU) have been indispensable in their research.
As an example, this research group initially used the block copolymers of polyethylene glycol/polyaspartic acid (PEG/PAA) in which a synthesized retinoid LE540 was encapsulated. Retinoid has received attention as a compound with various bioactivities, including anticancer effects, and many studies on synthesized retinoids have been conducted. In Dr. Sakurai's sample, synthesized retinoids were encapsulated in polymeric micelles composed of PEG as a shell and PAA as a core. “Several clinical studies are being conducted on polymeric micelles as DDSs of anticancer drugs. However, a detailed examination of the condition of encapsulated drugs has yet to be conducted,” explains Dr. Sakurai. Ultra-diluted samples encapsulated in sealed cells were placed in a vacuum chamber and SAXS measurements were conducted. LE540 addition caused the crystal structures of aspartic acids in the cores to become amorphous. This change, which is not induced by LE540 addition under normal conditions, suggests that the transformation phenomenon occurs in a tiny area inside a 6-nm radius. This result was published in Langmuir (2010).
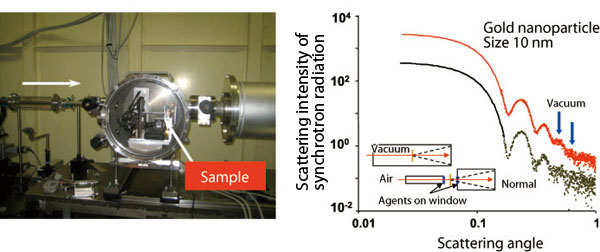
Scattering X-rays from solution cells in a vacuum are detected in a vacuum chamber that can accommodate up to six samples. Positions and transmission intensities are externally controlled and automatically measured. Right shows scattering data from gold colloids (10 nm). Noise level in this experiment (red dots) decreases by about ten-fold in the scattering angle range indicated by blue arrows compared to the existing setup (green dots).
Factors Affecting the Expression Efficiency of Gene Transfer Agents
Additionally, gene transfer agents comprised of lipid micelles were analyzed. Gene transfer agents are utilized in gene therapy to incorporate DNA and deliver it to genes in desired cells. This gene therapy, which is an application of DDSs, is expected to provide therapeutic effects for constitutional symptoms such as allergies.
Dr. Sakurai and colleagues used SAXS at SPring-8 to study the relationship between the composition of gene transfer agents and gene transfer efficiency. In particular, the effects of the composition of gene transfer agents on the probability of DNA being transferred into target cells were examined. The gene transfer efficiencies were measured at various solubilities and biocompatibilities by mixing various proportions of neutral lipids, specifically dioleoyl phosphatidylcholine (DOPC) and dilinoleoyl phosphatidylcholine (DLPC), with cationic lipids, benzylamine derivatives (BAs). Gene transfer agents with a high transduction efficiency (Fig. 3A) had hexagonal cylindrical structures prior to adding DNA. However, the addition of DNA increased the regularity of the structures. In contrast, agents without a transduction efficiency (Fig. 3B) had spherical micelle structures prior to adding DNA, but after adding DNA, the agents were converted into hexagonal cylindrical structures and were eventually transferred to lamellar structures composed of alternating layers of hydrophilic and hydrophobic phases. “DNA was transferred into the hydrophilic phase in cases with a high transduction efficiency (Fig. 2A), but was transferred into the hydrophobic phase in cases with a low transduction efficiency (Fig. 2B). We speculated that this difference is responsible for the expression efficiency of gene transfer agents,” explains Dr. Sakurai. Thus, the transduction efficiency of gene transfer agents strongly depends on the state of the neutral lipids, which is related to the micellar structures. “Visualization of nanoparticles, which was once impossible, can extensively promote the realization of nano-DDSs,” describes Dr. Sakurai.
Dr. Sakurai received the Society of Polymer Science of Japan (SPSJ) Mitsubishi Chemical Award in 2008 for his contributions, including those explained here as well as his research on DDSs using polysaccharides. SPSJ honored him by recognizing that his research on nano-DDS particles is a basic technology in polymer science that will contribute to industry.
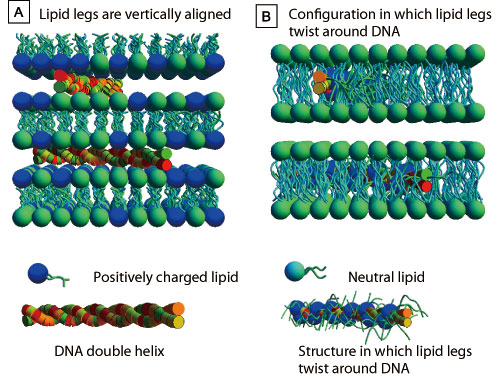
A: DNA in the hydrophilic phase caught between the head parts of two-molecule membranes comprised of cationic lipids. Dr. Cyrus R. Safinya and colleagues proposed this model. B: Lipid alkyl chains twisting around DNA hydrophobizes DNA, which is then captured in the hydrophobic phase. Dr. Victor A. Bloomfield and colleagues proposed this model.
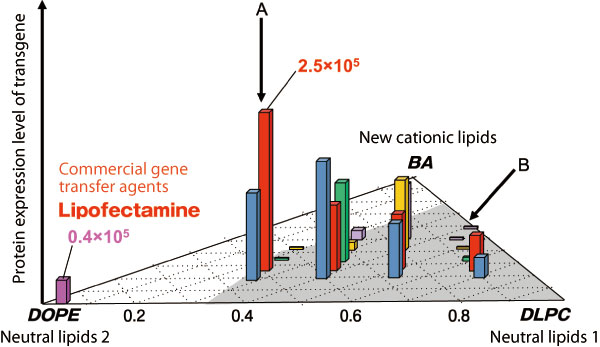
Composition of lipids and gene expression efficiencies (vertical axis) are depicted on a triangular correlation plot. Plane coordinates plot the composition ratio among BAs, dilinoleoyl phosphatidylcholine (DLPC), and dioleoyl phosphatidylcholine (DOPE) of neutral lipids. Composition ratio of BA:DOPE:DLPC is 1:2:1 for the case with the highest expression efficiency (indicated by A), whereas the composition ratio of BA:DLPC is 1:1 for the case with nearly zero expression (indicated by B), which we speculate correspond to A and B in Fig. 2, respectively.
References
1. K. Koiwai, K. Tokuhisa, Y. Kudo, S. Kusuki, Y. Takeda and K. Sakurai; Bioconjugate Chem., 16, 1349 (2005)
2. I. Akiba, N. Terada, S. Hashida and K. Sakurai; Langmuir, 26, 7544 (2010)
3. M. Sakuragi, S. Kusuki, E. Hamada, H. Masunaga, H. Ogawa, I. Akiba and K. Sakurai; J. Phys: Conference Ser., 184, 012008 (2009)