Topic 11: Determining the Structure of Frontier Carbon Materials
Mysterious Polyhedral Groups in the Nanoworld - Unraveling their True Characters
Fullerenes and carbon nanotubes have attracted much attention as promising raw materials for next generation nanotechnologies. At the same time, basic research aiming to explore their characteristics and establish application technologies of these new raw materials has been advancing. However, the true characters of fullerenes and nanotubes have been elusive. A fullerene is a soccer ball shaped molecule composed of 60 or more carbon atoms, while a carbon nanotube is a long and thin tubular material composed of a rolled hexagonal net-like graphene sheet. Hence, highly brilliant X-rays with extremely short wavelengths, which can brightly light the nanoworld on the order of 1/1,000,000 mm, are indispensable to reveal their characters.
Fullerenes and Nanotubes - Promising Raw Materials
A fullerene (C60), which was discovered by Richard E. Smalley, Harold W. Kroto, Robert F. Curl, (who shared the Nobel Prize in Chemistry in 1996) and two others in 1985, is unique due to its soccer ball shape. This molecule, which is composed of pentagonal and hexagonal molecular chains, was named fullerene after a famous architect, Buckminster Fuller, who designed geodesic domes consisting of pentagonal and hexagonal panels. Since its discovery, many have claimed to identify variations of fullerenes such as spheroid-shaped C70 and those containing metal atoms inside (Fig. 1A). However, even ten years after the discovery of fullerenes, the fundamental question about the existence of endohedral metallofullerenes remained, “Do they actually contain metal atoms inside?”
In 1991, Dr. Sumio Iijima1) (Researcher, NEC Tsukuba Research Laboratories, Japan) discovered a carbon nanotube (Fig. 2). A carbon nanotube, which is composed solely of carbon, is a tubular shape with a radius about 1/10,000 of that for a strand of hair (Fig. 2). Due to their electrically and physically superior properties nanotubes have attracted attention as fundamental materials in nanotechnology. Carbon nanotubes are expected to replace silicon devices, which are rapidly approaching their size reduction limit, as next generation electronic raw materials. However, the development of highly precise conductivity control technologies is indispensable to realize these next generation devices.
1) Currently Professor at Meijo University, Japan.
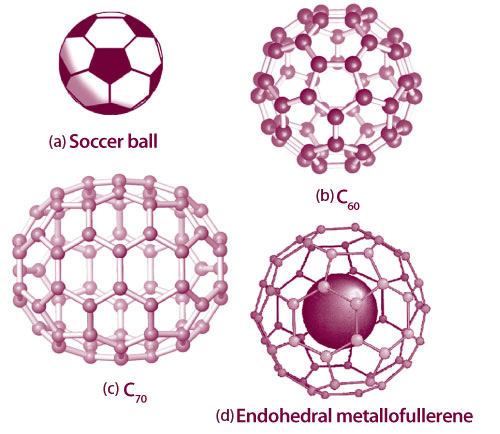
(a) Soccer ball. (b) Pentagon surrounded by five hexagons. (c) Spheroid-shaped fullerene. (d) Endohedral metallofullerene enclosing a single metal atom.
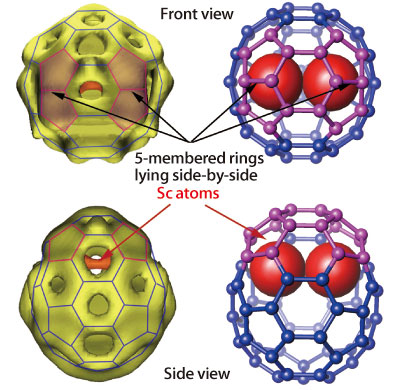
Fig. 1B. Electron density and structure model of Sc2@C66.
Unconventional structure of a fullerene is revealed.
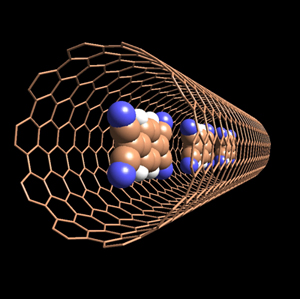
Electrons, which are supplied from the inside organic molecules including various fullerenes, carry electricity to the outer tube, creating a tubular conductor. Carbon nanotubes in which fullerenes are doped are called “pea pods” due to their shape.
Revealing the True Characteristics of Endohedral Metallofullerenes
Using synchrotron radiation at SPring-8, Dr. Makoto Sakata2) (Professor, Nagoya University, Japan), Dr. Masaki Takata3) (Associate Professor, ditto), and colleagues directly observed that a fullerene consisting of 82 carbon atoms (C82) actually contains a metal atom, yttrium (Y). They published their crucial data in 1995. This fullerene is termed Y@C82, where “@” indicates “endohedral.” Subsequently, their team has revealed the structures of fullerenes containing other metals such as scandium (Sc) and lanthanum (La), which are denoted as Sc@C82, Sc2@C84, Sc3@C82, La@C82, La2@C80, and Sc2@C66.
The powder X-ray diffractometer installed at the Powder Diffraction Beamline (BL02B2) at SPring-8 and the MEM/Rietveld method4) that Dr. Takata and colleagues developed have contributed significantly to their research. Even atoms and molecules beyond the scope of visible light can be observed using X-rays because the wavelength of an X-ray is equivalent to the size of an atom. Hence, analyzing the data obtained from the diffraction measurements of irradiated X-rays can visualize images of atoms and molecules.
In 2000, Dr. Hisanori Shinohara (Professor, Nagoya University) and colleagues created a new endohedral metallofullerene, Sc2@C66 (containing 2 Sc atoms) (Fig. 1B) in their effort to refute a traditional theory about the conformation of stable fullerene molecules, the isolated pentagon rule (IPR). IPR5)6) postulates that, “In fullerenes composed of multiple combinations of pentagonal and hexagonal carbon atom coupled rings (5- and 6-membered rings), none of the pentagons are in contact with each other. In such a conformation of the pentagons, some electrons are left over and do not hold the atoms together, causing the structure to become unstable. Thus, a single pentagon must be surrounded by five hexagons.” Through a stringent structure determination of Sc2@C66, Dr. Takata and colleagues successfully refuted IPR. They found that in this fullerene, the spherical structure containing two metal atoms (Sc) is significantly deformed, allowing two 5-membered rings to come into contact at two locations (Fig. 1B). This major breakthrough has removed limitations in designing the structures of fullerenes for semiconductors and superconductors as well as expanded the possibilities in the development of various raw materials in nanotechnology. Their research was published in Nature (November 2000) and received headline media coverage.
2) Currently Professor Emeritus at Nagoya University, Japan.
3) Currently Chief Scientist at RIKEN, Japan.
4) A revolutionary structural analysis technique that can determine the detailed atomic configurations of a material with an unknown structure based on the rough structure model of the material.
5) Kroto, H. Nature 329, 519-531 (1987).
6) Schmalz, T. G., Seitz, W. A., Klein, D. J. & Hite, G. E. J. Am. Chem. Soc. 110, 1113-1127 (1988).
Leading the Way toward Nanointegrated Circuits (Nano-ICs)
“We were very excited about what kinds of unknown phenomena would occur if organic molecules were to be doped (inserted) into nanotubes,” explains Dr. Yoshihiro Iwasa7) (Professor, The University of Tokyo, Japan), who along with his colleagues in 2003 were the first to successfully control the electric conductivity of carbon nanotubes by doping organic molecules into them. Their research group doped various organic molecules into nanotubes and analyzed the functions and structures of the doped nanotubes using the powder X-ray diffractometer at BL02B2. Their analysis revealed that electrons move from inserted organic molecules to the carbon nanotubes. They also found that changing the type or number of molecules to be doped can precisely control the properties and electric conductivity of nanotubes without restrictions. Their achievement, which has boosted the development of next generation IC elements, was published in Nature Materials (October 2003), and a 3D structural view of a carbon nanotube appeared on its cover (Fig. 2). A new type of nanostructures, termed “pea pods,” has been recently created by doping endohedral metallofullerenes into nanotubes, which look like frog eggs. Hence by varying the type of endohedral metallofullerene to be doped, the dream of designing nanocircuits with various electric properties is now reality.
Furthermore, Dr. Iwasa, Dr. Takata, Dr. Yasuhiro Takabayashi (chief researcher, Ideal Star, Inc., Japan), and colleagues have conducted collaborative research on endohedral metallofullerenes. Their research has revealed that a new fullerene (Cs3@C60), which is created by doping three cesium (Cs) atoms, is an insulator under ordinary pressures, but upon pressurization becomes a superconductor at 38 K (the highest superconducting transition temperature (Tc) achieved in a molecular superconductor). Their finding was published in Nature Materials (July 2008). However, the underlying mechanisms of how Cs3@C60 becomes a superconductor only under high pressures were unknown.
Hence, their research group then focused on elucidating the insulator to superconductor mechanism. They conducted experiments using the High Pressure Research Beamline (BL10XU) at SPring-8 to unravel the mystery. They demonstrated that Cs3@C60 is in a Mott insulator state (a singular state in which electrons are bound by the fullerene itself and cannot move) under normal pressures, but the electrons are released from this restriction to metalize Cs3@C60, which develops superconductivity at high Tc when pressurized (Fig. 3). Their accomplishment was published in the online edition of Science (March 2009). Dr. Iwasa has expressed his aspiration, “Now that the superconductivity mechanisms of fullerenes have been elucidated, we would like to promote the syntheses of high performance electronic elements based on this advancement.”
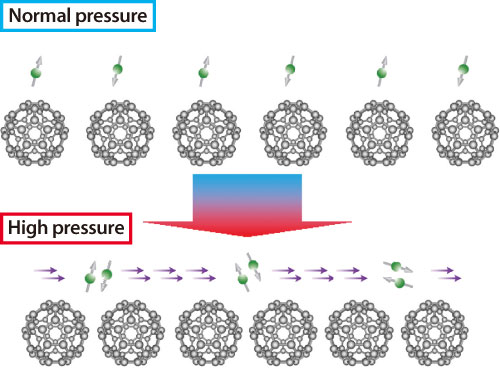
Under normal pressures, intermolecular distances between neighboring C60 molecules are relatively large, and electrons cannot move across the gap and remain on each molecule. This state is called the Mott insulator state. When high pressure is applied, neighboring C60 molecules come closer, allowing electrons to leap to neighbors. Simultaneously a strong attractive force is produced between electrons to create electrons pairs, resulting in superconductivity.
References
1. C.-R. Wang, T. Kai, T. Tomiyama, T. Yoshida, Y. Kobayashi, E. Nishibori, M. Takata, M. Sakata and H. Shinohara; Nature, 408, 426 (2000)
2. T. Takenobu, T. Takano, M. Shiraishi, Y. Murakami, M. Ata, H. Kataura, Y. Achiba and Y. Iwasa; Nature Materials, 2, 683 (2003)
3. A. Y. Ganin1,1, Y. Takabayashi, Y. Z. Khimyak, S. Margadonna, A. Tamai, M. J. Rosseinsky and K. Prassides; Nature Materials, 7, 367 (2008)
4. Y. Takabayashi, A. Y. Ganin, P. Jeglic, D. Arcon, T. Takano, Y. Iwasa, Y. Ohishi, M. Takata, N. Takeshita, K. Prassides and M. J. Rosseinsky; Science, 323, 1585 (2009)