Successful Structural Determination of Vault in Higher Organisms Using SPring-8 Beamline (Press Release)
- Release Date
- 16 Jan, 2009
- BL44XU (Macromolecular Assemblies)
|
A joint research team (team leader: Tomitake Tsukihara, specially appointed professor) comprising members from the Picobiology Institute (Department of Life Science, University of Hyogo), the Institute for Protein Research (Osaka University), and other institutes purified the largest reported intracellular macromolecular vault from rat liver and succeeded in the determination of its steric structure using a SPring-8 beamline, Macromolecular Assemblies Beamline BL44XU (contract beamline of the Institute for Protein Research of Osaka University). This achievement was reported in the American journal Science on 16 January 2009. Publication: |
Research background and achievement
A giant protein, a vault, often observed in the cells of higher organisms, is considered to transport materials similarly to a basket. A cancer-cell vault envelopes the medicine administered to a patient and makes it ineffective. When a person becomes infected with Pseudomonas aeruginosa, the vault promotes the intake of the germ into lung epithelial cells; the infected patient is protected from Pseudomonas aeruginosa by sterilization through the natural immune response of the epithelial cells.
Many researchers have carried out research on the vault transport mechanism since vault particles were discovered in 1986; however this mechanism has remained unclarified. Professor Tsukihara (Picobiology Institute, Department of Life Science, University of Hyogo) and his colleagues determined the vault structure at the atomic level and concentrated on the mechanism of the vault function. A vault is a giant protein consisting of a hundredfold to a thousandfold more atoms than regular proteins; thus the structural analysis involves many difficulties. This time, the joint research team succeeded in the determination of its steric structure using a SPring-8 beamline, Macromolecular Assemblies Beamline BL44XU (contract beamline of the Institute for Protein Research of Osaka University) used for structural analysis of biological macromolecular assemblies, such as giant proteins.
The vault is shaped like a rugby ball with a full length of 67 nm and a diameter of 40 nm at the center. A vault halved at the center of the molecule consists of 39 major vault proteins (MVPs) (Fig. 1). The vault outer shell consists of 78 MVPs, is very thin, and 2-3 nm thick, and the vault has a large cavity at its center. The cavity is so large that it can contain several 70S ribosomes, which are the largest intercellular molecules already structurally identified (Fig. 2). We can easily imagine that the vault can transport molecules in cells by enclosing them inside the cavity. The analysis of the MVP structure revealed that some of the MVPs have a structure unique to proteins that accumulate in a specific membrane called the lipid raft. This finding agrees with the results reported by a research group at Harvard University (Science 317, 130 (2007)), indicating that the vault promotes the intake of Pseudomonas aeruginosa via a lipid raft of the lung epithelial cells. This finding triggered the research on clarifying the vault function from its structure.
The structural determination of such a giant particle is difficult because few reliable high-resolution diffraction intensity data are obtained in the structural analysis. In fact, it was not possible using X-rays from bending magnet beamlines to obtain data sufficient for structural analysis. Thanks to SPring-8 beamline BL44XU with a high- brilliance X-ray source and large detectors, reliable data of 3.5 Å resolution were obtained and used to determine the structure. The success of the structural determination of a giant particle as a whole in vivo without destruction will considerably contribute to the future research of structural biology.
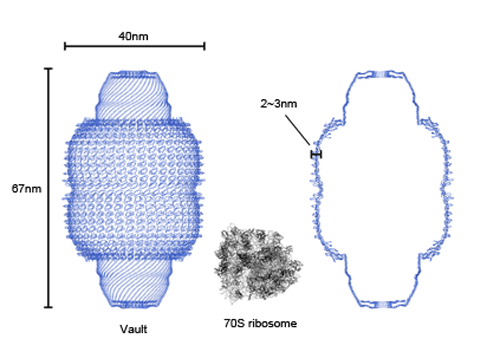 Fig. 2 Complete structure of rat liver vault
Fig. 2 Complete structure of rat liver vaultThe left and right figures show the complete structure of the vault and the cross section, respectively. For reference, the 70S ribosome, the largest intercellular organ among the molecular structures already structurally identified, is shown at the center. As revealed, the vault is sufficiently large to contain several 70S ribosomes.
|
For more information, please contact |
- Current article
- Successful Structural Determination of Vault in Higher Organisms Using SPring-8 Beamline (Press Release)
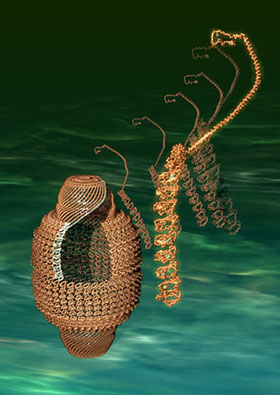 Fig. 1 Vault (left) and its constituent MVP molecules (right)
Fig. 1 Vault (left) and its constituent MVP molecules (right)
 .
.