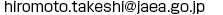Elucidation of the Structural Basis for Acceptor-Substrate Recognition of a Glucosyltransferase Involved in the Biosynthesis of Blue Flower Pigments -First Observation of the Anthocyanidin-Bound Form of the Enzyme- (Press Release)
- Release Date
- 26 Feb, 2015
- BL38B1 (Structural Biology III)
February 26, 2015
Japan Atomic Energy Agency
National Agriculture and Food Research Organization
Key points
• Crystal structures of Ct3GT-A and its unstable acceptor-substrate bound forms, involved in the biosynthesis of blue flower pigments, were determined.
• Further developments of Ct3GT-A based on this structural information will contribute to the enzymatic production of novel organic pigments or pharmaceutical candidates.
|
A research group at the Quantum Beam Science Center of Japan Atomic Energy Agency (JAEA; president, Shojiro Matsuura.) in collaboration with the NARO Institute of Floricultural Science of National Agriculture and Food Research Organization (NARO; president, Tokio Imbe.) succeeded in determining the crystal structures of a glucosyltransferase*1 involved in the biosynthesis of blue anthocyanins*2 which are known as natural pigments concerned in determining the color of flowers. Publication: |
<<Figures>>
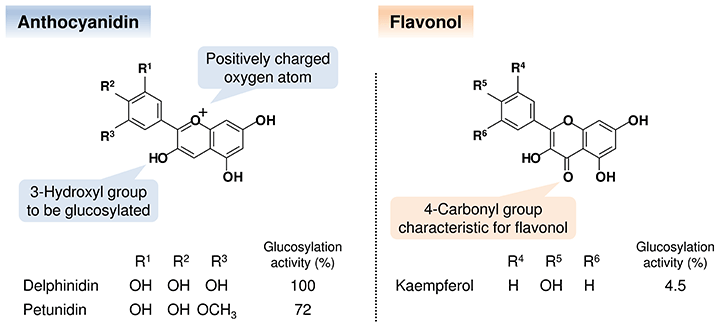
The delphinidin aglycone, which is one of the typical anthocyanidins, is converted into ternatin C5 through glucosylation by Ct3GT-A, Ct3GT-B, and Ct3′5′GT.
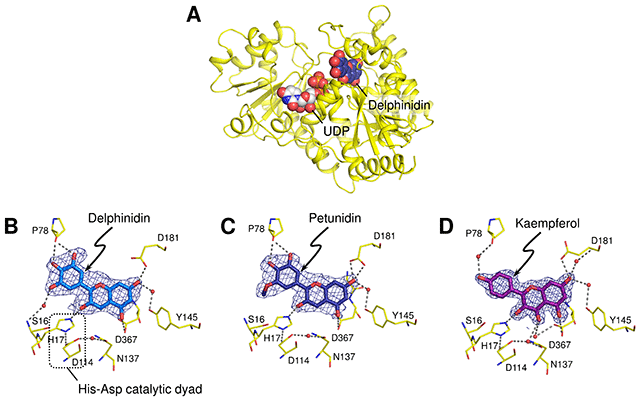
The substituent pattern on the single ring influences the color appearance of each glucoside. The glucosylation activities for petunidin and kaempferol were normalized against that of delphinidin.
The kaempferol molecule was situated in the binding site in a manner similar to that seen in the delphinidin- and petunidin-bound forms, although the glucosylation activities against flavonols were significantly low. It suggests that the activities depend on the deprotonation efficiency of the hydroxyl group to be glucosylated.
<<Glossary>>
*1 Glucosyltransferase
Glucosyltransferase is an enzyme that catalyzes the transfer of the glucosyl moiety from an activated nucleotide sugar, known as the "donor" substrate, to a nucleophilic glucosyl "acceptor" molecule, the nucleophile of which can be oxygen-, carbon-, nitrogen-, or sulfur-based.
*2 Anthocyanin
Anthocyanin is one of natural pigments located in all tissues of higher plants, including flowers, leaves, roots, and fruits, and serves to produce their characteristic color appearing red, purple or blue. Anthocyanin is a general term for the glucoside counterpart of anthocyanidin.
*3 Ct3GT-A
"Ct3GT-A" is an abbreviation of UDP-glucose: anthocyanidin 3-O-glucosyltransferase found in the petals of C. ternatea. The homologous enzymes to Ct3GT-A, identified in the same plant, were designated as Ct3GT-B and Ct3′5′GT based on each glucosylation activity.
*4 Anthocyanidin
Anthocyanidin is a general term for the tricyclic compound based on flavylium cation which is a type of oxonium form (Fig. 2). The different substitution patterns on the single ring of anthocianidins affect their color appearance.
*5 Photon Factory (PF)
PF in KEK, located in Tsukuba City, Ibaraki Prefecture, Japan, is the first synchrotron light source in Japan, which produce the X-ray regions. In this research, the PF Structural Biology Beamline (BL6A) was used for collecting the X-ray diffraction data.
*6 SPring-8
SPring-8, located in Harima Science Park City, Hyogo Prefecture, Japan, is a large synchrotron radiation facility, which delivers the most powerful synchrotron radiation currently available. The name "SPring-8" is derived from Super Photon ring-8 GeV (8 giga electron volts, being the power output of the ring). In this research, the Structural Biology Beamline III (BL38B1) was used for collecting the X-ray diffraction data.
|
For more information, please contact: Dr. Naonobu Noda (NARO) |
- Current article
- Elucidation of the Structural Basis for Acceptor-Substrate Recognition of a Glucosyltransferase Involved in the Biosynthesis of Blue Flower Pigments -First Observation of the Anthocyanidin-Bound Form of the Enzyme- (Press Release)