|
A research group led by researchers of Lund University (Sweden) and Technical University of Denmark succeeded in visualizing the intramolecular electron transfer reaction in a model compound of the photosynthesis using the beamline 3 (BL3) X-ray free-electron laser (XFEL) at the SPring-8 Angstrom Compact Free Electron Laser (SACLA) facility. The research was carried out in cooperation with the Institute of Materials Structure Science under the High Energy Accelerator Research Organization, RIKEN, and Japan Synchrotron Radiation Research Institute (JASRI).
The photosynthesis of plants starts when a pigment in leaves, called chlorophyll, absorbs optical energy that causes an electron in the chlorophyll to be removed and transferred into another molecule. The process from the absorption of optical energy by chlorophyll to the electron transfer is one of the most important processes during the early stage of photosynthesis. It occurs in an extremely short duration of ~1 ps [one picosecond (ps) is one trillionth of a second] and remains a mystery.
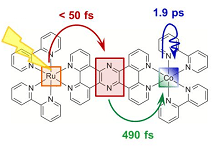
The research group used a molecule containing ruthenium and cobalt as a model compound of electron transfer in chlorophyll. It was observed that an electron is removed from ruthenium, moves through the molecule, and is transferred into cobalt when the molecule is irradiated with visible light for a short time (0.1 ps). The electron transfer between metal atoms and the change in the molecular structure associated with the electron transfer can be examined in detail by X-ray emission spectroscopy*1 and X-ray diffuse scattering,*2 respectively. In this study, time-course analysis was carried out by simultaneously performing X-ray emission spectroscopy and X-ray diffuse scattering after the irradiation of the molecule with visible light. The results indicate that an electron is transferred into cobalt approximately 0.5 ps after the start of irradiation, causing the cobalt to change from trivalent to bivalent. In addition, it was clarified that the molecular structure around the cobalt atom changes after another 2 ps of irradiation.
These results are expected to be helpful not only in understanding the photosynthesis of plants, but also in the development of a photosynthesis method to artificially convert optical energy into chemical energy by mimicking the photosynthesis reaction. The research group is also actively carrying out research and development of photocatalysts for use in artificial photosynthesis with sunlight on the basis of this method.
This research was supported by the X-ray Free Electron Laser Priority Strategy Program of the Ministry of Education, Culture, Sports, Science and Technology. The achievements were published online on 2 March 2015 in the scientific journal Nature Communications.
Publication:
Nature Communications 6, 6359 (2015)
Title:"Visualizing the non-equilibrium dynamics of photoinduced intramolecular electron transfer with femtosecond X-ray Pulses"
Authors: Sophie E. Canton, Kasper S. Kjær, György Vanko, Tim B. van Driel, Shin-ichi Adachi, Amélie Bordage, Christian Bressler, Pavel Chabera, Morten Christensen, Asmus O. Dohn, Andreas Galler, Wojciech Gawelda, David Gosztola, Kristoffer Haldrup, Tobias Harlang, Yizhu Liu, Klaus B. Møller, Zoltán Németh, Shunsuke Nozawa, Mátyás Pápai, Tokushi Sato, Takahiro Sato, Karina Suarez-Alcantara, Tadashi Togashi, Kensuke Tono, Jens Uhlig, Dimali A. Vithanage, Kenneth Wärnmark, Makina Yabashi, Jianxin Zhang, Villy Sundström & Martin M. Nielsen
DOI : 10.1038/ncomms7359
|