Topic 27: Investigating Mechanisms of Heavy Metal Hyperaccumulator Plants
Exploring the Secrets of Plants that Decontaminate Heavy Metal Contaminated Soils
Some plants absorb heavy metals from the environment, and accumulate them at high concentrations during the growth process. Reports have documented plants with heavy metal concentrations 300 times higher than that of the surrounding soil. Not surprisingly, the idea of removing heavy metals in contaminated soil and restoring the environment using plants, which is called phytoremediation, has recently received much attention. However, to apply this special phenomenon to practical environmental decontamination technology, the behavior of heavy metals in the plant bodies must be understood. Thus, the distributions and chemical forms of heavy metal elements must be elucidated at the cellular level. Fortunately, the high-energy X-ray microbeam at SPring-8 has made this possible. This topic reports cutting-edge research achievements at SPring-8.
Observing the Elemental Content in Live Plants
Soils contaminated with arsenic, lead, cadmium, chromium, or radioactive elements are problematic in various parts of the world. Phytoremediation is a pollutant reduction treatment in which harmful heavy metal elements are removed from the soil by reaping plants that absorb these elements. Compared to existing decontamination technologies, phytoremediation uses less energy, is more economical, and environmentally friendly. Thus, the potential demand for phytoremediation is huge.
However, plants with a hyperaccumulation ability for heavy metals are very limited. For example, the first land plant hyperaccumulator, Pteris vittata (Pteridaceae), was only identified in 2001 as an arsenic hyperaccumulator plant. Since then the hyperaccumulation ability for zinc and cadmium has been found in Arabidopsis halleri (Brassicaceae) in Japan. Thus, the application of heavy metal hyperaccumulator plants to phytoremediation is just beginning. Before hyperaccumulator plants can be identified and applied for soil decontamination, many questions must be answered. These include determining where and how these plants accumulate heavy metals, and why hyperaccumulators can absorb higher concentrations of heavy metals.
In April 2003, Dr. Izumi Nakai (Professor, Tokyo University of Science, Japan), Dr. Akiko Hokura1) (Assistant Professor, ditto), and colleagues initiated research to answer these questions using X-ray imaging. They studied the heavy metal hyperaccumulation ability of various plants, and decided to focus on Pteris vittata in Japan after meeting Dr. Nobuyuki Kitajima (Fujita Corporation, Japan), who had conducted a soil decontamination project using Pteris vittata. To elucidate the mechanisms of its arsenic hyperaccumulation ability, analysis with the resolution of 1 μm (μm = 10-6 m) was required. “SPring-8's Trace Element Analysis Beamline (BL37XU), which provides a combination of X-ray microbeams and X-ray fluorescence analysis, was the only facility that could perform element analysis and chemical form analysis at the cellular level,” explains Dr. Nakai.
1) Currently Associate Professor at Tokyo Denki University, Japan.
Elucidating the Transition of Arsenic to Leaves
When X-rays are irradiated onto a sample, an inner-shell electron of the constituent atom in a sample is excited or emitted out, and an outer-shell electron moves down to the resultant hole2) . Then a fluorescent X-ray whose energy is determined by the difference between the energy levels of these two electrons is subsequently emitted. Because each element has a specific fluorescent X-ray energy, the constituent elements can be identified from the obtained spectrum (wavelength-dependent intensity distribution). Additionally, the element concentration can be obtained from the fluorescent X-ray intensity. This analysis technique is called X-ray fluorescence (XRF). However, the spatial resolution is limited to 10 μm in conventional XRF using non-directional X-rays and not synchrotron radiation, making it difficult to obtain the complex behavior of elements in plant tissues. To overcome this difficulty, an X-ray microbeam was prepared by exploiting the highly brilliant and directional synchrotron radiation to measure the 2D distributions of contained elements. This is called synchrotron radiation microbeam X-ray fluorescence (SR-μ-XRF).
SR-μ-XRF can measure live plants and fresh tissues, but investigating the elemental distribution at the tissue/cellular level requires sample manipulations. These manipulations prevent shrinkage and tissue deformation due to drying as well as the migration and outflow of contained elements during the measurements. Along with the aid of botanical experts, Dr. Nakai and colleagues have been accumulating the know-how about plant cultivation and sample preparation. For example, to maintain the structure of a sample at the cellular level, it must be quickly frozen to prevent ice crystals from forming in the cells and to maintain the sample shape. Through this frozen technique, XRF imaging of samples in a near-living state has become possible, allowing the anatomical positional relationship between the element distributions and the tissue structure to be measured.
In addition to freezing the sample, sample thickness is very important. If the thickness of a section is ~150 μm, several cells will overlap, preventing the elemental distributions within a single cell to be determined. Fortunately, their research group has successfully prepared 10-0-μm thick frozen sections.
After these careful preparations, they were ready to measure Pteris vittata at SPring-8 (Fig. 1). Pteris vittata was grown under optimal conditions with the help of Dr. Kitajima and Dr. Tomoko Abe (RIKEN, Japan). After immersing Pteris vittata in an arsenic solution for a given length of time (30 min or 24 h), frozen sections were resected from the leaves. Scanning the samples at 3-μm intervals every 0.3 sec was realized using the X-ray microbeam with a beam cross-section of 2 × 2 μm2. In this manner, the changes in the arsenic distribution were visualized with time (Fig. 2).
Surprisingly, arsenic reaches the marginal regions where it is stored 30 min after administration, which is amazingly fast. Additionally, they found that arsenic initially accumulates in the conductive tissues, and is then preferentially distributed to paraphyses and false indusium.
2) This process is called a transition.
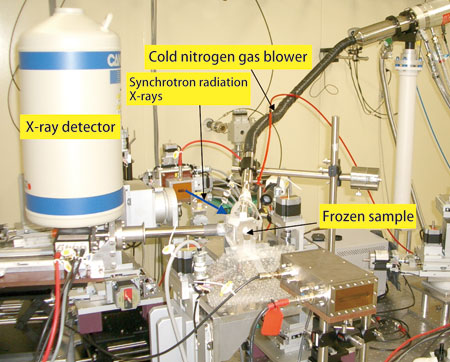
BL37XU allows researchers to analyze frozen sections of Pteris vittata in a near-living state.
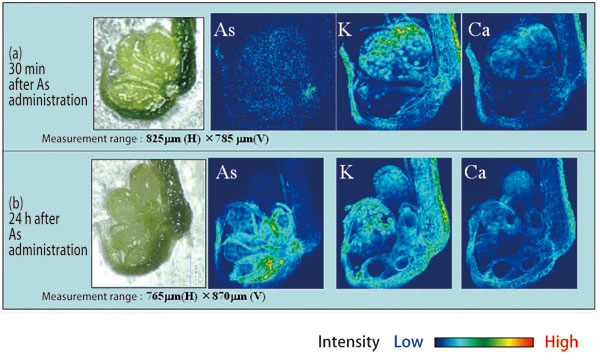
(a) Distribution of elements 30 min after arsenic administration. Arsenic reaches the base of the sporangium of Pteris vittata's pinna in 30 min.
(b) Distribution of elements 24 h after arsenic administration. Arsenic concentrates in the base of the sporangium, but the entrance into a spore is suppressed.
Precisely Analyzing Cadmium Contained within Rice
In addition to studying arsenic-accumulating plants, Dr. Nakai's research group has studied various cadmium-accumulating plants, such as Arabidopsis halleri and Athyrium yokoscense. Because cadmium has a larger atomic number than arsenic, high-energy synchrotron radiation at SPring-8 is indispensable to excite inner-shell electrons of cadmium. Dr. Yasuko Terada (Senior Scientist, the Japan Synchrotron Radiation Research Institute) and colleagues were the first to conduct SR-XRF analyses using high-energy X-rays with a 1-μm beam size at BL37XU. Currently BL37XU at SPring-8 is the only experimental facility in the world where cadmium is examined at the cellular level.
In Arabidopsis halleri's leaves, cadmium along with zinc and manganese accumulate in the trichome, which is a capillary outgrowth that consists of tiny cells with ~20-μm diameter. SR-XRF using the X-ray microbeam revealed that cadmium accumulates in the joint of trichome. Additionally, X-ray absorption fine structure (XAFS) analysis revealed that cadmium is bonded to oxygen or nitrogen.
In Japan, the cadmium content in brown rice is regulated by law, but the actual analyses of a very small quantity of cadmium in plants are cumbersome and difficult. However, high-energy SR-XRF can easily measure the cadmium distribution in brown rice. Their research group also examined the 2D distribution of cadmium within rice, and found that cadmium is uniformly distributed over the edible parts of white rice (endosperm) obtained from rice plants grown with an additional 1 ppm of cadmium. Their work indicates that polishing rice does not remove cadmium (Fig. 3).
As just described, their research group was the first to reveal where arsenic and cadmium reside in plants as well as their chemical forms at the cellular level. “High-energy SR-XRF is quite powerful in analyzing heavy metal elements in plants. We will continue to elucidate the accumulation mechanisms of heavy metals in heavy metal hyperaccumulator plants using synchrotron radiation,” comments Dr. Nakai as he talks about the future.
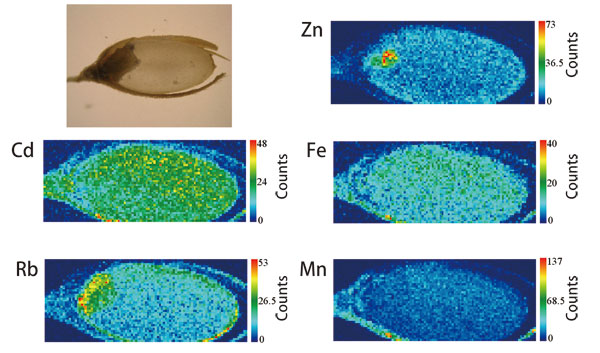
Zinc, an essential element, concentrates in the germ, while cadmium is uniformly distributed over the endosperm, which is consumed as white rice.
Reference
1. R. Onuma, A. Hokura, N. Kitajima, I. Nakai, Y. Terada, T. Abe, H. Saito, Shigeo Yoshida; Proc. 8th Int. Conf. X-ray Microscopy, IPAP Conf. Series, 7, 326 (2006)