Topic 12: Elucidating the Mechanisms by which 12CaO·7Al2O3 with a Cage Structure is Transformed into the Metallic Phase
Changing Cement Raw Materials into a Metal-like Conductor
All materials can be categorized broadly as a conductor and an insulator in terms of their electric properties, but the boundary between these two categories is not definite due to the existence of a semiconductor, which exhibits properties of both. Materials such as cement raw materials are categorized as insulators. However, Dr. Hideo Hosono (Professor, Tokyo Institute of Technology, Japan), Dr. Yoshiki Kubota (Associate Professor, Osaka Prefecture University, Japan), Dr. Masaki Takata (Chief Scientist, RIKEN, Japan), and colleagues successfully converted cement raw materials into conductors. Additionally, they revealed the conversion mechanisms using synchrotron radiation at SPring-8, and were the first to develop new conductors using common materials as raw materials.
Can Cement Raw Materials, which Are Insulators, Really Be Converted into Conductors?
About 99% of the Earth's crust is composed of the following eight elements: oxygen, silicon, aluminum, iron, calcium, sodium, potassium, and magnesium. All the aforementioned elements, except oxygen, exist as an oxide. Most oxides are in the form of light metal oxides and are common raw materials in glass, cement, and ceramics. It is a common knowledge that they are not electrically conductive.
Dr. Hosono and colleagues have been unraveling the crystal structures of various light metal oxides, which are known as insulators, on the nanometer level (nm = 10-9 m). By skillfully utilizing their knowledge of these nanostructures, their research group has been investigating the conversion of light metal oxides, which are originally insulators, into semiconductors or conductors (metals). “I have been keeping my eye on light metal oxides for a long time. Long-term studies on glasses and ceramics have taught me that there must be something hidden behind them. Then I came up with a research idea about combining light metal oxides and electrons,” explains Dr. Hosono.
Lime (CaO), a compound composed of calcium and oxygen, and aluminum oxide (Al2O3), a compound comprised of aluminum and oxygen, are representative insulators that commonly appear in textbooks. In 2003, Dr. Hosono and colleagues successfully converted one of the constituent materials of cement, 12CaO·7Al2O3 (C12A7), which is composed of CaO and Al2O3, into a semiconductor. However, C12A7 remained semiconductive, and could not be converted into the metallic state, which defied common knowledge. It is known that if a semiconductor such as silicon is doped (injected) with more electrons, the conductivity continually increases until the doped electron density exceeds some specific value until it is eventually converted to the metallic state. Thus, Dr. Hosono and coworkers continued to make small strides toward converting C12A7 into the metallic state, but definite conclusions remained unclear.
Drastic Conversion of C12A7, an Insulator, into the Metallic State
C12A7 is a crystal composed of interconnected nanosized cages that contain oxygen ions (O2-) (Fig. 1). Dr. Hosono and his research group focused on the fact that although the oxygen ions remain in the cages, the ions move across the interconnected cages of C12A7 when the temperature exceeds 700 °C. In their experiments, C12A7 was enclosed in a glass tube with titanium metal, which can produce stable compounds by capturing the moving oxygen ions but does not react with the C12A7 cages themselves. The tube was subsequently heated to 1,100 °C. Titanium was selected because a reaction with C12A7 destroys the cages, enabling almost 100% of the oxygen ions in the cages to be replaced by electrons from titanium. This replacement can be controlled so that C12A7 is converted from an insulator to a semiconductor, and eventually into the metallic state (Fig. 2).
The following two observations confirmed metallization of C12A7. First, the electric resistance decreases as the temperature decreases. (The electric resistance of semiconductors should increase as temperature decreases). Second, upon adding a small amount of impurities with magnetism, the electric resistance does not vary monotonically with temperature, but instead has a minimum at a specific temperature. This latter phenomenon is called the Kondo effect, which is induced by the interactions between magnetic impurities and conduction electrons.
This metallized C12A7 has a high conductivity; the conductivity is at the same level as manganese metal and is twice that of graphite. When a normal semiconductor such as silicon is converted into a metal, the number of conduction electrons increases, but the average electron mobility per electron decreases. However, their research revealed that the average electron mobility of metallized C12A7 is more than ten times higher than that of semiconductive C12A7 (Fig. 3, top).
To investigate the reason for this phenomenon, they conducted structural analyses of C12A7 using a powder X-ray diffractometer at the Powder Diffraction Beamline (BL02B2) at SPring-8 and the MEM/Rietveld method. The MEM/Rietveld method, which was developed by Dr. Takata and colleagues, is an innovative structural analysis technique to determine the detailed atomic structures of materials based on a rough structure model of a material with an unknown structure. The MEM/Rietveld method was developed by combining electron density imaging and powder diffraction pattern fitting.
According to Dr. Hosono, “The shapes of cages are deformed in the insulator state when oxygen ions are trapped inside these nanosized cages. However, as we decrease the number of oxygen ions by replacing them with electrons, this deformation is gradually released, and a perfect shape is recovered for all the cages when the electron density exceeds a specific value. Then the electrons suddenly are free to move, and the semiconductor becomes a metal (Fig. 3, bottom right). The high precision diffraction data obtained by utilizing highly brilliant X-rays at SPring-8 revealed the detailed mechanisms for the structural conversion from the insulator state into the metallic state. The unique property of C12A7 is that it is chemically stable but similar to potassium metal, easily releases electrons. I presume that electronic devices based on this property will be realized in the not-so-distant future.”
Transparent metals such as indium (a rare metal) are indispensable to manufacture LCDs. If this research advances, commonly available elements (Dr. Hosono calls them “ubiquitous elements”) could replace rare metals. In particular, 70% of visible light can penetrate a 100-nm thick C12A7 film. Their research achievements were published in Nano Letters (April, 2007). Furthermore, three months after successful metallization of C12A7, Dr. Hosono and colleagues converted C12A7 into a superconductor, and have since discovered another new iron-based high-temperature superconductor.
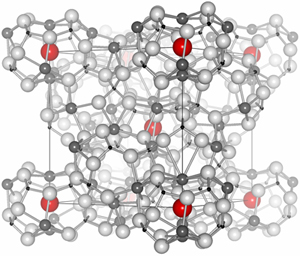
Each cube represents the unit cell where each cell is comprised of 12 cages, and only two of which contain oxygen ions (red).
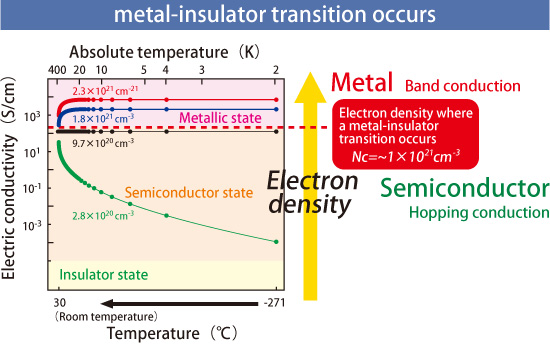
Electron doping induces metallization of C12A7 (insulator).
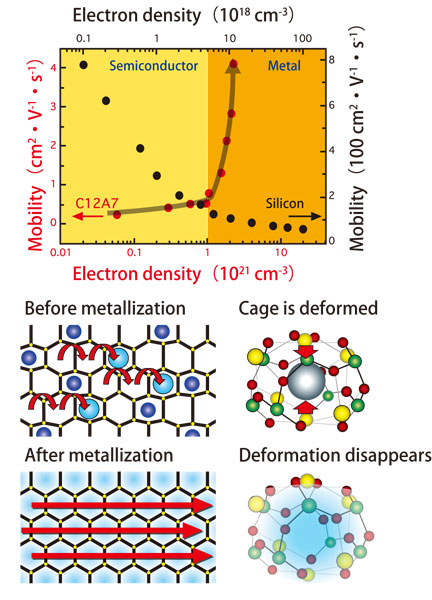
Top: Contrary to conventional semiconductors, electrons can move easily when C12A7 becomes a metal. The grey sphere represents an oxygen ion.
Bottom: State changes in C12A7 when an oxygen ion in the cage is replaced by electrons. Red arrows represent the movement of conduction electrons.
Reference
1. S. W. Kim, S. Matsuishi, T. Nomura, Y. Kubota, M. Takata, K. Hayashi, T. Kamiya, M. Hirano and H. Hosono; Nano Lett., 7, 1138 (2007)