Topic 26: Elucidating Dioxin Production Mechanisms in Refuse Incineration
Revealing Why Dioxins Are Produced Even in Perfect Combustion
Dioxins, many of which are highly toxic, are produced in various processes, including combustion processes in metal refining and chlorine bleaching of paper. The major source of dioxins is speculated to be the imperfect combustion during combustion processes in refuse incinerators. Recently, refuse incinerators have adopted high-temperature combustion (> 800 °C) with the goal of reducing the amount of dioxin produced through perfect combustion, followed by rapid cooling of the exhaust gas, and dioxin absorption via activated carbon. Despite these efforts, dioxins are still being produced. However, research conducted at SPring-8 has elucidated the production mechanisms of dioxins, and this cutting-edge research has provided insight to eliminate dioxin production.
Role of Copper Compounds in Dioxin Production
Polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) are called dioxins. However, there are 75 types of PCDDs and 135 types of PCDFs. Dioxins are a collective name for 210 chemical compounds with a common structure where one to nine chlorine atoms are connected to two benzene rings that sandwich oxygen atoms. The skeletal structure, binding site, and number of chlorine atoms all determine the toxicity of a particular dioxin.
Various measures have been sought to reduce the production of dioxins. Recently, perfect combustion and temperature control in refuse incinerators have been implemented to reduce dioxin production. Unfortunately, dioxins are resynthesized during the cooling processes of exhaust gas in refuse incinerators. Exhaust gas contains smoke dust or fly ashes. In the cooling process of fly ashes, the resynthesis of dioxins is initiated, and it is maximized at 300-400 °C and terminated below 200 °C. The reaction involves a catalyst. Numerous experiments have demonstrated that copper, which is found in copper wires, substrates, and paints in refuse, forms copper compounds, and these copper compounds play a catalytic role in the resynthesis of dioxins. However, the reaction mechanisms of the catalytic actions have yet to be elucidated.
“We aimed to thoroughly explore how copper compounds contribute to dioxin production,” explains Dr. Masaki Takaoka (Associate Professor, Kyoto University, Japan), who majored in urban environmental engineering. He has been conducting research to prevent the resynthesis of dioxins during the cooling processes of refuse incinerators and consequently, to reduce the amount of dioxin produced. To prevent dioxin resynthesis, the detailed mechanisms of the resynthesis must be elucidated. In other words, the following must be determined: 1) the role of copper compounds in the resynthesis of dioxins during the cooling processes of fly ashes, and 2) the types of copper compounds and the mechanisms promoting the resynthesis.
XAFS - Most Promising Technique to Decipher the Secret of Dioxin Resynthesis
To elucidate the dioxin resynthesis mechanisms, researchers have great expectations for the XAFS Beamline (BL01B1) at SPring-8. XAFS is an abbreviation for X-ray absorption fine structure. At BL01B1, the absorbed X-ray dose by a sample is measured by continuously varying the energy of the incident X-rays that are irradiated onto a sample. In this energy scan measurement, the absorbed X-ray dose sharply increases at a specific energy called the absorption edge. The absorption edge varies by element, and fine variations in the distribution of absorbed X-ray dose are observed near the absorption edge. This distribution is called the absorption spectrum, which reflects the atomic structure of a material.
XAFS analyses occur in two separate energy regions: 1) near the peak of the absorption edge and 2) at some distance above the absorption edge. The fine structure in the former is called the X-ray absorption near edge structure (XANES), while that in the latter is called the extended X-ray absorption fine structure (EXAFS). Because XANES largely depends on the electron states of the atoms of a specific element in a sample, the following information can be obtained: the valence values, elemental species, and content rates of X-ray absorbing atoms. On the other hand, the interference between the waves of photoelectrons emitted from X-ray absorbing atoms and those scattered by neighboring atoms induces EXAFS; thus, the distance of the X-ray absorbing atoms from the neighboring atoms and the number of the latter (coordination number) can be obtained.
In October 2000, Dr. Takaoka and colleagues conducted experiments at SPring-8 to examine the copper contained in fly ashes extracted from incinerators. Fly ash samples were prepared by reproducing the temperature and gas conditions equivalent to those in actual incinerators in their university's laboratory, and they analyzed their samples at SPring-8. However, the results were unsatisfactory. To overcome this problem, they conducted further experiments in October 2003. They encapsulated fly ash in a glass container, and the temperature and gas conditions were maintained so that they were equivalent to actual incinerators to directly observe the real-time reactions of the ashes (Fig. 1).
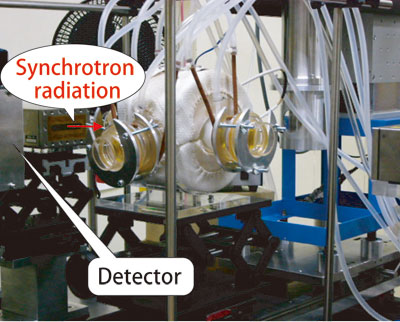
Fly ashes were encapsulated in a white container and irradiated by synchrotron radiation. Measurements were performed by setting the inside temperature of the container to that of an incinerator.
Chlorine Removed from Copper Compounds Becomes a Raw Material of Dioxins
Dr. Takaoka's group conducted the following experiments. 1) First, X-rays were irradiated onto samples at various temperatures, 2) then the obtained spectra were compared to those of a standard reference material, and 3) finally the content of various copper compounds were estimated at each temperature. These experiments revealed that the major component of the copper compounds contained in incineration ashes is atacamite, which is a compound of copper(II) chloride (CuCl2) and copper(II) hydroxide (Cu(OH)2).
Heating atacamite initiates the reduction of copper at ~200 °C, which is also the temperature where the synthesis of dioxin begins. Upon further heating, copper exists mainly as copper(I) chloride (CuCl) at 300 °C, but at 400 °C, CuCl2 decreases and copper(II) oxide (CuO) is produced. The complex oxidation-reduction reactions depend on temperature.
On the other hand, an oxidative chlorination reaction, termed oxychlorination, was observed in the reduction process of copper. This is a reaction where carbon compounds contained in fly ashes are chlorinated. Past studies have suggested that organochloride compounds, which have a slightly simpler structure than dioxins, are also produced. Dr. Takaoka and colleagues' results support this assertion.
Consequently, they found that chlorine coupled to copper can be decoupled, and used as a raw material for dioxins (Fig. 2). In fact, the dioxin content increased more than 500-fold from 84 ng (ng = 10-9 g) per 1 g of fly ash at room temperature to 4,900 ng at 400 °C. These experiments were repeated using various mock fly ashes. Not all cases produced CuCl (Fig. 3: mock fly ash B). “This finding indicates that adjusting the conditions can terminate the copper catalytic cycle and control the chemical reactions. Thus, preventing CuCl formation reactions may suppress dioxin production,” notes Dr. Takaoka. Their achievement was published in Environmental Science & Technology (August 2005), which is a well-established journal in environmental science and engineering. Additionally, their findings were spotlighted at an X-ray analysis discussion meeting held at Kyoto University in October 2005.
Their research group is conducting further experiments using mock fly ashes to accumulate data about the relationship between dioxin production and the chemical changes of chlorine, copper, and other metals as well as researching the effects of chemical suppressors. These suppressors are highly effective in the laboratory; thus, after overcoming some technical issues, suppressors may be applicable in an optimized process for the entire exhaust gas processing system.
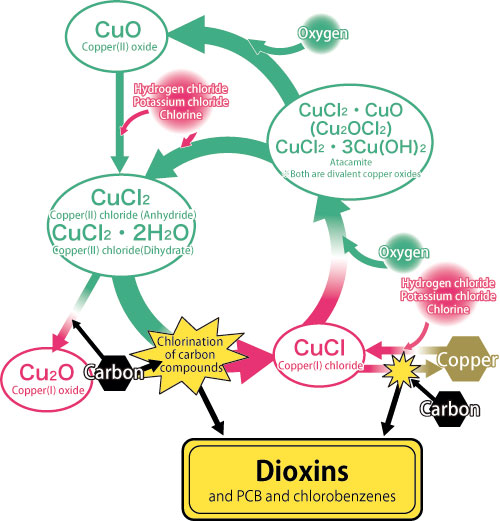
During the reduction process of copper(II) chloride (CuCl2) by carbon, chlorine leaves CuCl2 to make copper(I) chloride (CuCl). In this case, chlorine is added to carbon, and partly makes dioxins. However, chlorine leaves CuCl, and in some cases couples with carbon. Additionally, CuCl reacts with oxygen in the atmosphere to produce divalent copper compounds (and copper(II) oxide (CuO) is produced in some cases). These divalent copper compounds react with chlorides or chlorine in the surrounding environment to produce CuCl2. This cycle is repeated to produce dioxins.
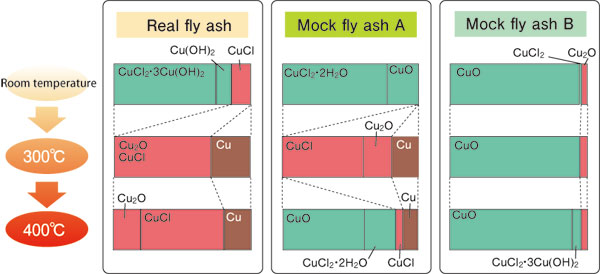
Compositional changes of copper compounds contained in combustion fly ashes from room temperature to 300-400 °C. ■ indicates univalent copper compound, while ■ indicate divalent copper compounds. For mock fly ash B, few univalent copper compounds are observed.
Reference
1. M. Takaoka, A. Shiono, K. Nishimura, T. Yamamoto, T. Uruga, N. Takeda, T. Tanaka, K. Oshita, T. Matsumoto and H. Harada; Environ. Sci. Technol., 39, 5878 (2005)