Biology
List of Application Documents
Click the No. of the document to view the procedure for each document.
Download the document by clicking the download icon.
| No. (U-Form) | Document Name |
Download
|
How to write?
|
| Conducting an experiment using specific organisms | |||
| 4-1-1c(19-1) | Notification of a plan for an experiment using specific organisms | ||
| Free format | Manual for safe handling | ||
| 4-1-2c(19-2) | Notification of changes in the plan for an experiment using specific organisms | ||
| 4-1-3c(19-3) | Notification of transfer of level 2 specific biological samples | ||
| 4-1-4c(19-4) | Notification of storage of level 2 specific biological samples | ||
| 4-1-5c(19-5) | Notification of disposal of level 2 specific biological samples | ||
| 4-1-6c(19-6) | Indication of storage of specific biological samples | ||
| Free format | Record of inspection and restoration | ||
| 4-1-7abc | Report of completion of the plan for an experiment using organisms |  |
|
| Conducting a genetic modification experiment | |||
| e4-2-1abc(20-1) | Application form for a genetic modification experiment | ||
| e4-2-2abc(20-2) | Genetically modified organisms carry in/out notification form | ||
| e4-2-3abc(20-3) | Report of genetic modification experiment (progress/completion) | ||
| e4-2-4abc(20-4) | Notification of storage place of genetically modified organisms | ||
| e4-2-5c(20-5-3) | Application for Genetic Recombination Experiment Worker and Letter of Pledge |
||
| e4-2-6c(20-6-3) | End of the Engagement in Genetic Modification Experiment | ||
| e4-2-7abc(20-7) | (Registration/Deregistration) of Genetic Modification Experiment Worker and Implementation Report on Educational Training | ||
| e4-2-8abc | Information based on the Law Concerning the Conservation and Sustainable Use of Biological Diversity through Regulations on the Use of Living Modified Organisms. | ||
| Conducting an animal experiment | |||
| e4-3-1c(17-5) | Application form for approval of an animal experiment (experiment using radiation) | ||
| 4-3-2c(17-6) | Application for registration and agreement form for an animal experiment worker | ||
| 4-3-3c(17-7) | Report of completion of an animal experiment | ||
| 4-3-5ac | Verification form | ||
| Online | Notification of experimental animal delivery schedule | ||
| Online | Notification of experimental animal delivery schedule | ||
| e4-3-6 | Record of the use of a mobile/sectional animal treatment room | ||
| Online | Use application of Cell Culture Room | ||
| Online | Use Application for Cell Culture Room | ||
| Conducting an experiment using human material | |||
| 4-4-1abc(23-1) | Agreement regarding research using human material | ||
| 4-4-2abc(23-2) | Application for use of human material | ||
| Conducting an experiment using specific parts of cows | |||
| Free format | “Application for use of specific cow parts” and “Application for use of specific cow parts” | ||
| Conducting an experiment using prohibited imports | |||
| <Act on Domestic Animal Infectious Diseases Control> |
|
||
| e4-6-1 | Application for Permission to Import Prohibited Articles (Act on Domestic Animal Infectious Diseases Control) | |
|
| e4-6-2 | Animal quarantine briefing materials (Act on Domestic Animal Infectious Diseases Control) | ||
| e4-6-3 | Testing and research plans (Act on Domestic Animal Infectious Diseases Control) | ||
| e4-6-4 | Manual(Act on Domestic Animal Infectious Diseases Control) | ||
| <Plant Quarantine Law> | |||
| e4-7-1 | Application for Import Plant Inspection(Plant Quarantine Law) | ||
| <importation procedure for mice and rats in accordance with the notification system for animal importation> |
|||
| e4-8-1 | Conducting an experiment requiring “importation procedure for mice and rats in accordance with the notification system for animal importation” | ||
| Cabinet Order for Enforcement of the Invasive Alien Species Act | |||
| e4-9-1 | Management Record Books on the Circumstances of Breeding, Etc. of Designated Invasive Alien Species (Bull Frogs) | ||
| e4-9-2 | Inspection Record Books on Bull Frog Breeding Conditions and Specified Breeding Facilities | ||
Application Procedures for Public and Contract Beamline Users
Conducting an Experiment Using Specific Organisms
Conducting an Experiment Using Microorganisms (Bacteria, Viruses, Fungi, Parasites, etc.)
The following procedures for “conducting an experiment using specific organisms” are necessary when conducting an experiment in which pathogenic microorganisms (bacteria, viruses, fungi, infectious nucleic acids/plasmids, etc.), parasites, as well as materials that pose a hazard to the human body, livestock, and farm and marine products through biological interaction, such as toxic substances, carcinogens, and allergens that are produced by the said microorganisms (hereinafter referred to as “specific biological samples”), are used.
A separate procedure for “Conducting a genetic modification experiment” is necessary when genetically modified organisms will be used.
“Application for a user proposal”
· Conducting an experiment using specific organisms in the user project
“Procedures required from before the start of the experiment to the completion of the experiment”
· When handling a specific biological sample specified as Biosafety Level 1
· When handling a specific biological sample specified as Biosafety Level 2
<Reference>Biosafety Level (Japanese)
National Institute of Technology and Evaluation
https://www.nite.go.jp/nbrc/mrinda/list/
NATIONAL INSTITUTE OF INFECTIOUS DISEASE
https://www.niid.go.jp/niid/images/biosafe/kanrikitei3/Kanrikitei3_2020101-1.pdf
Application for Notification of the Plan for an Experiment Using Specific Organisms (For the Person in Charge of the Experiment)
(1) Specific biological samples that can be used
At Japan Synchrotron Radiation Research Institute (hereinafter referred to as “JASRI”), only samples of Biosafety Level 1 and 2 can be used.
(2) Applicable experimental facilities (laboratories where specific biological samples can be used)
· Experimental Hall of Storage Ring Facility (BSL-1 laboratory)
· Medium-length Beamline Facility (BSL-1 laboratory)
· Experimental Animal Facility (BSL-1 laboratory, BSL-2 laboratory)
It is necessary to get approval before using specific biological samples at JASRI. The following procedures should be followed by the person in charge of the experiment 1)
1) The person in charge of the experiment must be a person who has at least one year experience in handling microorganisms, and who will come to Japan Synchrotron Radiation Research Institute and actually participate in the experiment. Students are not acceptable.
‹Documents to be submitted›
· Form-No.1 Notification of a plan for an experiment using organisms
· Manual for safe handling (free format [Example]) should be submitted to JASRI Safety Office.
‹Deadline and the place where documents should be submitted›
Mail “Form-No.1 Notification of a plan for an experiment using organisms” separately to JASRI Safety Office for the semi-annual application for a user proposal.
*When planning an experiment, it should be adequately discussed with JASRI so that the experiment complies with the “standard of facility/equipment and safety of the laboratory” where the samples will be handled.
(4) Procedure flow
‹Review of safety›
Safety will be reviewed by a safety committee based on the submitted documents, taking the methods of handling as well as the aspect of the facility into account.
The person in charge of the experiment (a representative is acceptable) will be asked to attend the committee meeting to be interviewed regarding the content of the experiment.
‹Notification of approval›
The decision of approval or non-approval will usually be sent in writing within 1 month of review by the genetic modification experiment safety committee.
Procedures Required from Before the Start of the Experiment to the Completion of the Experiment (For the Person in Charge of the Experiment)
The procedures are different for cases in which specific organisms of Biosafety Level 1 are used and cases in which specific organisms of Biosafety Level 2 are used.
(1) Before the start of the experiment
| Using organisms specified as Biosafety Level 1 |
|
‹Start of experiment› ‹Training› ‹Start of experiment› |
| Using organisms specified as Biosafety Level 2 |
|
‹Healthcare of a biological experiment worker› ‹Delivery of specific biological samples› If the document has not been submitted 10 days before the delivery, samples such as microorganisms may not be able to be carried in to JASRI. ‹Storage of specific biological samples› ‹Start of experiment› ‹Training› ‹Inspection of equipment› |
(2) During the experiment
‹Sign›
The signs that are stipulated in Form No.6 must be placed near the entrance of the laboratory and the storage space of specific biological samples during the experiment. Contact the Safety Office if the signs are needed.
(3) After completion of the experiment
| Using organisms specified as Biosafety Level 1 |
|
‹Restoration› ‹Report of the completion of the experiment› |
| Using organisms specified as Biosafety Level 2 |
|
‹Carrying out of specific biological samples› If the document was not submitted 10 days before the delivery, it may not be possible to carry specific biological samples out of JASRI. ‹Disposal of specific biological samples› ‹Restoration› ‹Report of completion of the experiment› |
(4) Other procedures
‹Changes in the plan for a biological experiment›
When changing the specific biological samples and experiment workers which have been granted approval, submit Form No.2 Notification of changes in the plan for an experiment using specific organisms to the JASRI Safety Office at the time of semiannual application for a user proposal.
Conducting a Genetic Modification Experiment
The following procedures are necessary before conducting genetic modification experiments that are stipulated in the Law Concerning the Conservation and Sustainable Use of Biological Diversity through Regulations on the Use of Living Modified Organisms (Act 97, 2003). The delivery of genetically modified organisms is also included. It is also necessary the procedure for a genetic modification experiment even if the experiment includes only the use of genetically modified animals (genetic modification of the animals will not be conducted at JASRI).
The procedures for “Conducting an animal experiment” are also necessary.
Click here for the flow from the application to the completion of the genetic modification experiment (Japanese)
for the flow from the application to the completion of the genetic modification experiment (Japanese)
“Application for an experimental plan”
· When conducting a genetic modification experiment as a user project.
“Procedures required from before the start of an experiment to the completion of the experiment”
“Registration of a genetic modification experiment worker”
· JASRI staff *Japanese
· Users (Radiation worker)
Application for the Approval of a Genetic Modification Experiment (For the Person in Charge of the Experiment)
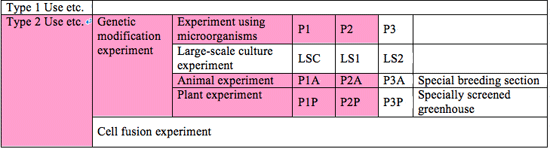
(1) Experiments that can be conducted at Japan Synchrotron Radiation Research Institute (hereinafter referred to as “JASRI”)
The ranges of experiments that can be conducted at JASRI are as follows.
(2) Applicable experimental facilities (laboratories where specific biological samples can be used)
· Experiment Hall of Storage Ring Facility
· Medium-length Beamline Facility
· Experimental Animal Facility
(3) Application for an experiment
It is necessary to get approval before conducting a genetic modification experiment at JASRI. The following procedures should be followed by the person in charge of the experiment 1)
1) The person in charge of the experiment must be a person who has at least one year experience of conducting genetic modification experiments, who will come to JASRI and actually participate in the experiment. As a general rule, students are not acceptable.
‹Documents to be submitted›
Submit Form No.20-1 Application for a genetic modification experiment. [Example Animals,Microorganisms]
‹Deadline and the place where documents should be submitted›
Mail an “Application for approval of a genetic modification experiment” separately to JASRI Safety Office for semiannual application for a user proposal of Spring-8.
* When planning an experiment, make sure that the experiment complies with the Law Concerning the Conservation and Sustainable Use of Biological Diversity through Regulations on the Use of Living Modified Organisms (Act 97, 2003) as well as the applicable laws and regulations.
(4) Procedure flow
‹Review of safety›
Safety will be reviewed by the safety committee based on the submitted documents, taking the methods of handling as well as the aspect of the facility into account.
The person in charge of the experiment (a representative is acceptable) may be asked to attend the committee meeting to be interviewed regarding the content of the experiment.
‹Notification of approval›
The decision of approval or non-approval will usually be sent in writing within 1 month of review by the genetic modification experiment safety committee.
Procedures Required from Before the Start of the Experiment to the Completion of the Experiment (For the Person in Charge of the Experiment)
(1) Before the start of the experiment
‹Notification of the experiment worker and training for each experiment›The person in charge of the experiment must appoint experiment workers2) who will conduct the experiment, and have them participate in training before starting the experiment. When the training is completed, submit Form 20-7 Form for notification/notification of change and report of training of a genetic modification experiment worker to the JASRI Safety Office at least 10 days before the start of the experiment. 2) The person must have been registered as a genetic modification experiment worker.
‹Delivery of genetically modified organisms›
It is necessary to submit Form 20-2 Notification of genetically modified organisms (carry in/out) and receive confirmation from the Safety Office before delivering genetically modified organisms to JASRI.
Attach the “Information based on the Law Concerning the Conservation and Sustainable Use of Biological Diversity through Regulations on the Use of Living Modified Organisms3)”.
Submit the document at least 10 days before transfer to the JASRI Safety Office.
If the document was not submitted 10 days before the delivery, it may not be possible to carry such samples as genetically modified organisms into JASRI.
3) Make sure to obtain information from the organization from which the genetically modified organisms are delivered. Although the format is not specified, a document without adequate information will not be accepted. Refer to the “Information based on the Law Concerning the Conservation and Sustainable Use of Biological Diversity through Regulations on the Use of Living Modified Organisms” for details.
(2) During the experiment
‹Sign›
The signs that are stipulated in the laws must be placed at the laboratory, near the entrance of the laboratory, and the storage space of genetically modified organisms. Contact the Safety Office if the signs are needed.
‹Progress repor›
Annual submission of Form 20-3 Report of genetic modification experiment (progress/completion) [Example] is required during the course of the experiment. Confirm the timing of the submission with JASRI Safety Office.
‹Carrying out of genetically modified organisms›
It is necessary to submit Form 20-2 Notification of genetically modified organisms (carry in/out) and receive confirmation from the Safety Office before carrying out genetically modified organisms from JASRI.
Attach information based on the Law Concerning the Conservation and Sustainable Use of Biological Diversity through Regulations on the Use of Living Modified Organisms4). Mail this information beforehand to the person in charge at the organization to which the genetically modified organisms will be delivered.
Submit the document to JASRI Safety Office at least 10 days before carry in/out.
4) Although the format is not specified, a document without adequate information will not be accepted. Refer to the Form “Information based on the Law Concerning the Conservation and Sustainable Use of Biological Diversity through Regulations on the Use of Living Modified Organisms” [Example] for details.
(3) At the completion of the experiment
‹Notification of completion›
When completing the experiment, submit Form 20-3 Report of genetic modification experiment (progress/completion) [Example] to the JASRI Safety Office. If there is another plan for a new experiment and the storage of genetically modified organisms is necessary for future use, submit Form 20-4 Notification of storage place of genetically modified organisms [Example] as well. When using the stored genetically modified organisms, a new application for the experiment is required.
(4) Other procedures
‹Addition of experimental materials (donor nucleic acid, vector, host, etc.)›
When adding experimental material concerning the experiment requiring containment measures, submit Form 20-1 Application for a genetic modification experiment [Example Animals , Microorganisms] at the time of semiannual application for a user proposal.
‹Addition, elimination, and changes of experiment workers2)›
Submit Form 20-7 Form for notification/notification of change and report of training of genetic modification experiment worker [Example] as soon as the change is confirmed.
2) The person must have been registered as a genetic modification experiment worker
‹Changes in the place where the experiment will be conducted and genetically modified organisms will be stored›
Regarding the changes in the experiment requiring containment measures, submit From No.20-1 Application form for a genetic modification experiment at the time of semiannual application for user proposals.
‹Changing the person in charge of the experiment›
When changing the person in charge of the experiment, the experiment must be temporarily terminated. Submission of a new application as a new experiment is required.
(This rule is not applicable in case the person in charge of experiment is not able to perform the duty due to unavoidable circumstances such as travel, disease, or accident, and a previously appointed substitute will perform the duty.)
Registration of a Genetic Modification Experiment Worker (For the Person in Charge of the Experiment
Those who will participate in a genetic modification experiment must be registered as genetic modification experiment workers. Register as soon as possible, since registration requires many items such as training, a health check, and confirmation of documents.
(1) Procedures for registration
‹Required documents and the place where documents should be submitted›
JASRI staff must submit Form 20-5-1 (JASRI staff) Application for genetic modification experiment worker to the JASRI Safety Office.
Users (Radiation Workers) must submit Form 20-5-3 Application and agreement for genetic modification experiment worker to the JASRI Safety Office.
‹Training›
To be registered as a genetic modification experiment worker, participation in the training conducted by JASRI is mandatory.
‹Time required to complete the registration procedure›
Since the applicant will be registered as a genetic modification experiment worker only after the completion of the above-mentioned training and confirmation of the submission of required documents, it is recommended to commence the process of registration early.
(2) Renewal procedure
‹Validity period of experiment worker registration›
Since the registration of a genetic modification experiment worker for a user expires at the end of the fiscal year, renew the registration at the end of the fiscal year if the genetic modification experiment will continue into the following year. The renewal procedure is the same as the procedure for the initial registration.
(3) Procedure for experiment worker termination
‹Required documents and the place where the documents should be submitted›
If someone wants to cancel the genetic modification experiment worker registration in the middle of the fiscal year, submit Form 20-6-3 Notification of genetically modified experiment worker termination (users) [Example] to the JASRI Safety Office.
(4) Procedures for changes
<Changes in the content of registration>
When the organization (department etc.) of the experiment worker changes, terminate the registration and start a new registration process.
<On Genome Editing Technology>
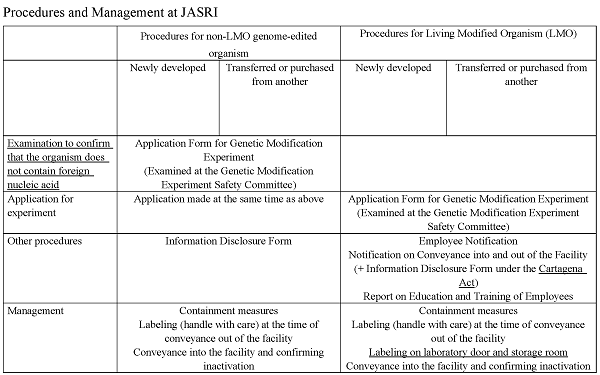
Living organisms obtained from genome editing technology (those without foreign nucleic acid), as defined in the “Note on the Use, Etc. of Living Organisms Obtained from the Use of Genome Editing Technology in the Research Phase” (Notification of the Director-General of the Research Promotion Bureau, Ministry of Education, Culture, Sports, Science and Technology, No.100, dated June 13, 2019), will require the following procedures to be taken in advance.
*If the living organism contains foreign nucleic acid, it will be treated as a living modified organism (LMO) as before.
■ Before starting an experiment on a living organism obtained by using genome editing technology (those without foreign nucleic acid) → Please submit the application form for approval on a genetic modification experiment.
■ Before transferring to another party or receiving from another party a living organism obtained by using genome editing technology (those without foreign nucleic acid) → Please provide information on such transfer of the living organism, etc.
Conducting an Animal Experiment
The following procedures are required before starting an animal experiment.
A flow chart of the process up to the start of the animal experiment. Click here for the flow from application to the completion of the animal experiment.
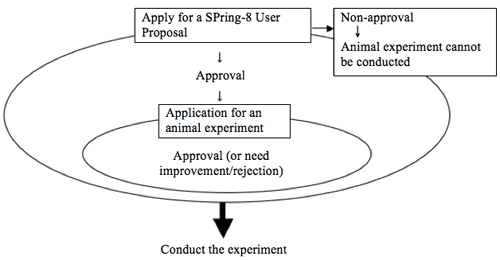
“Application for approval of the plan for an experiment”
Conducting an animal experiment for the user project
“Procedure from before the start of the experiment to the completion of the experiment”
“Registration for animal experiment worker” JASRI staff *Japanese
RIKEN staff (Only when working as a user) & Users (Radiation workers) here
Application for Approval of an Animal Experiment (For the Person in Charge of an Animal Experiment)
(1) Regarding the species of animals
The following animals can be used at Japan Synchrotron Radiation Research Institute (hereinafter referred to as “JASRI”).
Mouse, rat, guinea pig, hamster (all should be SPF animals)
Rabbit (should be a clean animal)
* Consult the person in charge to use other animals than the ones listed above.
(2) The place where an animal experiment should be conducted
The animal experiment can be conducted only in the places approved by JASRI animal experimentation committee.
Regarding the details of each facility, refer to the Biological sample preparation laboratory page.
Storage Ring Facility Experiment Hall
Medium-length Beamline Facility
Experimental Animal Facility
(3) Experimental Animal Facility
Experimental animal care* is only allowed in the places listed in the table below.
| Place for experimental animal care* | |
| Place for experimental animal care* | Mouse Facility |
| Rabbit Facility | |
| Aquatic Animals Facility |
* Animal care means... Keeping the animals temporarily at an Experimental Animal Facility
(4) Application for animal experiment
It is necessary to get approval before conducting an animal experiment at JASRI. The following procedures should be followed by the person in charge of the experiment1).
1) The person in charge of an animal experiment must be a person who has at least one year experience of conducting an animal experiment, who will come to JASRI and actually participate in the experiment. Students are not acceptable. The person in charge of an animal experiment does not have to be the same person as the one who is in charge of the experiment for the user project.
If the experimental animals are genetically modified organisms, separate application for a genetic modification experiment is required. Follow the Procedure for the genetic modification experiment.
When planning the animal experiment, make sure that the content of the experiment conforms with the “Regulations for Animal Experiments” , “Guideline for Proper Conduct of Animal Experiments, dated June 1, 2006 by the Science Council of Japan,” and related laws and regulations. Consult the persons in charge listed below for specific procedures concerning animal experiments and other questions.
(Person in charge)
Animal Experimentation Supervisor: Naoto Yagi
Deputy Animal Experimentation Supervisor:
Experimental Animal Facility Manager: Naoto Yagi
‹Required documents› [Conducting an animal experiment for a user project]
Form 17-5 Application for approval of an animal experiment (experiment using radiation)
(Reference) Domestic laws related to “Act on Welfare and Management of Animals”
SCAW (Scientists Center for Animal Welfare) Handbook-The Japanese Association of Laboratory Animal Facilities of National University Corporations, PDF
[Guideline for application 1---Radiation]
‹Deadline›
Perform semiannual application (the first term in December, the second term in July) for SPring-8 user proposals. If the proposal is accepted, mail “Form 17-5 Application for approval of an animal experiment” separately to the JASRI Safety Office.
‹Validity period of animal experiment plan›
The validity period of the approved animal experiment is until the end of the relevant radiation experiment.
(5) Procedure flow
‹Review of the animal experiment plan›
The content of the animal experiment will be reviewed by the JASRI animal experimentation committee based on the submitted documents. The person in charge of an animal experiment (a representative is acceptable) may be asked to attend the committee meeting to be interviewed regarding the content of the experiment.
‹Notification of approval›
The decision of approval or non-approval will usually be sent in writing within 1 month of review by the animal experimentation committee.
Procedures Required from Before the Start of the Experiment to the Completion of the Experiment (For the Person in Charge of the Animal Experiment)
(1) Before the start of the experiment
‹Registration for animal experiment worker and training›
The person in charge of an animal experiment must confirm that the person who has to conduct the animal experiment has been registered as an animal experiment worker at JASRI2). Those who have not been registered as animal experiment workers are not able to conduct animal experiments.
The person in charge of an animal experiment must provide training concerning the handling of experimental animals to the animal experiment worker before the start of the experiment. Make sure to keep a record of the training, such as the date, place, and content of the training.
2) The registration of an animal experiment worker must have been completed before the start of the experiment.
‹Delivery of experimental animals to JASRI›
Before delivering experimental animals to JASRI, perform the procedure found here “Notification of experimental animal delivery schedule.”
(2) During the experiment
‹Entering the place where the experiment takes place›
Make sure that no one besides the animal experimental workers enters the place where the animal experiment is conducted.
(3) At the completion of the experiment
‹Notification of completion›
As soon as the user project is completed, submit Form 17-7 Report of completion of an animal experiment to the JASRI Safety Office.
(4) Procedures for changes after the approval of an animal experiment
‹Addition of experimental materials (experimental animal species)›
As a general rule, the addition of animal species that were not in the plan is not allowed. If the addition of experimental animals is absolutely necessary, contact JASRI Safety Office as soon as possible.
‹Changing the place of the experiment›
As a general rule, changing the place of the experiment is not allowed. Adding another place for the experiment is not allowed, either. Contact JASRI Safety Office as soon as possible, if it is absolutely necessary to change the place of the experiment.
Registration of an Animal Experiment
Those who will participate in an animal experiment must be registered as an animal experiment worker. Register as soon as possible, since registration requires many items such as training, health check, and confirmation of documents.
(1) Procedures for registration
‹Required documents and the place where the documents should be submitted›
JASRI staff here *Japanese
RIKEN staff must submit Form 17-6 Application for the registration and agreement form for an animal experiment worker to the JASRI Safety Office. *Only when conducting an experiment as a Radiation Worker.
The user (Radiation Worker) must submit Form 17-6 Application for the registration and agreement form for an animal experiment worker to the JASRI Safety Office.
‹Training›
To be registered as an animal experiment worker, participation in the training concerning the animal experiments and experimental animals conducted by JASRI is mandatory. If participation in the training provided at the organization to which the person belongs is confirmed, the training at JASRI is not necessary.
The person in charge of the animal experiment must provide training concerning the handling of the experimental animals before the experiment.
‹Time required to complete the registration procedure›
Since the applicant will be registered as an animal experiment worker only after the completion of the training and the health check, it is recommended to commence with the process of registration early.
(2) Renewal procedure
‹Validity period of animal experiment worker registration›
As for a Radiation Worker who has been registered as an animal experiment worker using Form 17-6 Application for the registration and agreement form for an animal worker, the registration of the animal experiment worker expires at the end of the fiscal year. Renew the registration at the end of the fiscal year if the animal experiment will continue into the following year. The renewal procedure is the same as the procedure for the initial registration
(3) Procedures for changes
‹Changes in the content of the registration›
When the department or title of the animal experiment worker change, submit Form 17-6 Application for the registration and agreement form for an animal experiment worker to the JASRI Safety Office as soon as possible.
Conducting an Experiment Using Human Material
The following procedures are necessary to use human materials at Japan Synchrotron Radiation Research Institute (hereinafter referred to as “JASRI”) for research using radiation.
(1) Intended Human materials
Human materials include tissues, organs, cells, blood (whole blood and blood components), and other body fluids collected from living and dead human bodies. Human materials that are publicly available as specimens and research materials are excluded.
(2) Intended place for the experiment
· Storage Ring Facility
· Medium-length Beamline Facility
· Experimental Animal Facility
· RI Laboratory
· Storage Ring Annex West
(3) Procedure for carry in
‹Management system for human materials›
When using human materials for research, Management system must be put in place. The “human material management body” is an organization that controls the human materials used in the user project. The “person in charge of handling” is a member of the relevant user project (including the person in charge of the experiment), who belongs to the human material management body and is present at the SPring-8 to be responsible for the handling of human materials when the human materials are used during the project.
must be put in place. The “human material management body” is an organization that controls the human materials used in the user project. The “person in charge of handling” is a member of the relevant user project (including the person in charge of the experiment), who belongs to the human material management body and is present at the SPring-8 to be responsible for the handling of human materials when the human materials are used during the project.
‹Submission of Form 1 “Agreement regarding research using human material”›
This form is a document that pledges that the organization, (the dean or a person in a higher position and the Ethical Review Board of the organization) to which the person in charge of the experiment belongs, has approved the use of human materials in the project.
The person in charge of the experiment must submit this form for each project to the JASRI Safety Office.
‹Submission of Form 2 “Application for use of human material”›
This form is required for each human material. This is a document to notify selection of the “person in charge of handling” of the relevant human materials, and to pledge that the following items have been approved for each human material that will be used in the experiment, by the human material management body (the chairperson or a person in a higher position).
· Proper acquisition of human materials
The human materials are properly acquired* and controlled by the human material management body.
*When the human materials are collected at the human material management body,
→ The donor or the family member of the donor must provide a voluntary written agreement after receiving a clear explanation of the purpose of using the human materials.
* When the human materials are collected at another place than the human material management body,
→ In addition to agreement of the donor and a family member of the donor, the organization that collect the human materials must agree to supply the human materials to the human material management body.
· Approval of research
The human material management body must approve the radiation experiment using the relevant human materials.
(4) Notes for using human materials for research
· Those who conduct the experiment must pay adequate attention to the dignity and human rights of the donor when using and storing human materials.
· Those who conduct the experiment may not conduct any experiment beyond the range of approval of the donor, the family member of the donor and the organization which those who conduct the experiment belongs to.
· Make sure to lock the place where human materials are stored.
· It is not allowed to transfer or share the human materials at JASRI under any circumstances.
· The human materials must be returned to the human materials management body after completion of the experiment.
(5) Handling human materials that may be infectious
Follow theProcedure stipulated in the Biosafety Regulation.
(Example: The specimen should be treated at Biosafety Level 2. When contamination with microorganisms of Biosafety Level of 3 or higher is suspected, the specimen may not be carried in.)
Conducting an Experiment Using Specific Cow Parts
Before carrying in specific cow parts, a proper legal process must be taken. Submit a copy of the “application for use of specific cow parts” and “permission for use of specific cow parts.” Contact the Safety Office when applying for a permit including SPring-8.
■What are the specific parts?
They are the parts of the body where high levels of BSE-causing abnormal prions are distributed.
These include the head excluding the tongue and cheek meat (including the brain, eyeballs, and tonsils), spinal cord, and distal ileum (2 m before the cecum).
Conducting an Experiment Using Prohibited Imports
Animal quarantine (Act on Domestic Animal Infectious Diseases Control, Rabies Prevention Act, and Act on the Prevention of Infectious Diseases and Medical Care for Patients with Infectious Diseases)
To import items that are subject to animal quarantine (designated quarantine items), those items must pass an inspection conducted by a government agency of the exporting country corresponding to the Animal Quarantine Service in Japan and be accompanied by an inspection certificate issued by that government agency.
Generally, there is an agreement between the exporting and importing countries on the list of matters that must be inspected and certified in the exporting country, which are called the animal health requirements.
<Reference> Animal health requirements
http://www.maff.go.jp/aqs/english/
Plant Quarantine Law
To prevent the spread of pests from outside Japan that may cause substantial damage to agricultural production in Japan, the Plant Protection Act (Act No. 151 of 1950), Article 7, lists import-prohibited articles as shown below, and stipulates that “No person shall import imported-prohibited articles.”
Types of Import-Prohibited Articles
1. Quarantine Pests (those insects, mites, nematodes and other invertebrates, and vertebrates listed in the Regulation for Enforcement of the Plant Protection Act, Appended Table 1, No. 1-1 and 1-2 that are injurious to useful plants)·
2. Quarantine Plants (those fungi, slime molds, bacteria, parasitic plants, and viruses listed in the Regulation for Enforcement of the Plant Protection Act, Appended Table 1, No. 2-1 and 2-2 that are injurious to useful plants)
3. Soil or plants to which soil is attached
4. Plants listed in the Regulation for Enforcement of the Plant Protection Act, Appended Table 2 and Appended Table 2-2
5. Plants listed in the Regulation for Enforcement of the Plant Protection Act, Appended Table 1-2 (excluding those cultivated as indicated in the table)
The plants listed in Appended Table 1-2 require inspection in the exporting country for pests that would be difficult to detect at the time of import but would be easy to detect through inspection at the cultivated sites in the exporting country. These plants include those that were not inspected at the cultivation sites in the exporting country.
6. Containers and packaging for 1 and 5 above
Even for the above import-prohibited articles, there are exclusion clauses that enable import and use of import-prohibited articles if granted permission from the Minister of Agriculture, Forestry and Fisheries for use in testing and research or in special circumstances stipulated by ministerial ordinances (i.e. exhibition and storage as specimen in natural history museums, zoos, botanical gardens, aquariums, etc., use as evidence in criminal investigation, and breeding of melon flies at plant protection institutes for melon fly control).
Click here for a flowchart of the procedure (Japanese)
(Japanese)
Regarding the prohibited imports, ask the Safety Office directly.
Conducting an Experiment Requiring “Importation Procedure in Accordance with the Notification System of Animal Importation such as mice and rats”
Before carrying in animals requiring an importation procedure for mice, rats, etc., in accordance with the notification system of animal importation, a proper legal process must be taken.
Since September 1, 2005, it has been required that the importation of “live Rodentia, Lagomorpha, and other terrestrial mammals,” “live birds,” and “carcasses of Rodentia and Lagomorpha” should be notified without exception. A document that proves the absence of dangerous infection prepared by the trading partner is required. Refer to the following, Regarding the notification system of animal importation (Ministry of Health, Labour and Welfare), for the required procedures.
Regarding the importation of mice and rats, ask the Safety Office directly.
Conducting an Experiment Using Invasive Alien Species
Before carrying in invasive alien species, the proper legal process must be taken.
When keeping Specified Foreign Organisms (including plants) is necessary for a research experiment, application documents for the approval must be submitted to the Ministry of the Environment (and the Ministry of Agriculture, Forestry and Fisheries) beforehand.
Regarding the care of the Specific Foreign Organisms, the standard of the facility and the content of the application set by the relevant ministry are defined separately for each organism. Since universal management of all the species is not possible, individual control manuals for each species have been prepared at JASRI.